Having previously reported the synthesis of new bifunctional chiral phosphine-thiourea organocatalysts A, B and C, derived from L-proline, and their applications in asymmetric [3+2] cyclisation between ethyl-butan-2,3-dienoate and N-benzylidene-p-toluenesulfonamide; we have demonstrated, in this project, that these compounds catalyzed efficiently asymmetric allylic substitution of tert-butoxycarbonyloxy-MBH adduct with phthalimide. Good yields and enantioselectivities were observed.

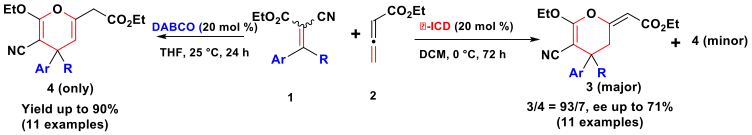
We have also described the first example of phosphine-thiourea organocatalyzed C–S bond formation affording enantioenrichied α-methylene-β-mercapto esters in good yields and with enantiomeric excess up to 93%. In collaboration with the Prof. Sasai’s group (Osaka University), and supported by Sakura and Core-to-Core Programs (Japan-European Program), we have reported the first [4+2] annulation of allenoate and all-carbon tetrasubstituted alkenes catalyzed by an amine catalyst for the synthesis of highly functionalized 2H-and 4H-pyran derivatives. These results will open opportunities in synthesis of bioactive heterocyclic products and other pharmaceutical products.
The main part of our research activities is based on the valorization of easily accessible and inexpensive biomass compounds (isosorbide, isomannide, natural amino acids such as L-proline, (S)-pyroglutamic acid, ...). The transformation of these compounds under 'Green Chemistry' conditions, namely, solvent-free synthesis under microwaves activation method, allowed us to prepare (reversible) functionalized chiral ionic liquids, multi-dentate ligands (amino-alcohols, diamines, phosphines,...), chiral N-heterocyclic carbenes and functionalized chiral organocatalysts. These compounds have shown excellent activities in asymmetric metal or organic catalysis. Part of the group's research activities concerns the synthesis of therapeutic products (natural or unnatural) and their derivatives for QSAR studies. Some application examples are presented below. It should be noted that during this five-year period, many international and national collaborations have been established.
Asymmetric organocatalysis
Having previously reported the synthesis of new bifunctional chiral phosphine-thiourea organocatalysts A, B and C, derived from L-proline, and their applications in asymmetric [3+2] cyclisation between ethyl-butan-2,3-dienoate and N-benzylidene-p-toluenesulfonamide; we have demonstrated, in this project, that these compounds catalyzed efficiently asymmetric allylic substitution of tert-butoxycarbonyloxy-MBH adduct with phthalimide. Good yields and enantioselectivities were observed.
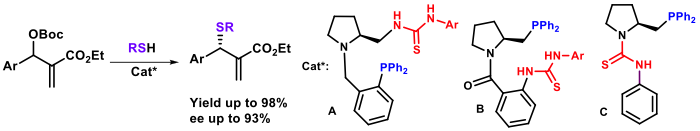
We have also described the first example of phosphine-thiourea organocatalyzed C–S bond formation affording enantioenrichied α-methylene-β-mercapto esters in good yields and with enantiomeric excess up to 93%. In collaboration with the Prof. Sasai’s group (Osaka University), and supported by Sakura and Core-to-Core Programs (Japan-European Program), we have reported the first [4+2] annulation of allenoate and all-carbon tetrasubstituted alkenes catalyzed by an amine catalyst for the synthesis of highly functionalized 2H-and 4H-pyran derivatives. These results will open opportunities in synthesis of bioactive heterocyclic products and other pharmaceutical products.
![]()
G. Vo-Thanh, Prof; M. Toffano, CR CNRS; C. Bournaud, MCF; T.-T.-D. Ngo, T.-H. Nguyen, PhD students, Vietnamese Government Scholarships-Program 332 and 911
Chiral ligands and NHCs: Design, synthesis and applications in asymmetric catalysis
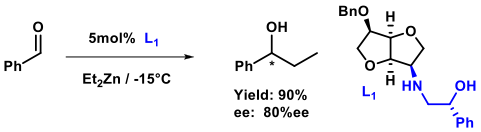
During the past five years, some new families of ligands (amino alcohols, diamines, phosphines, ...) were designed and synthesized from isosorbide and isomannide as optically pure, inexpensive biomass starting materials. The transition metal complexes formed with these ligands are evaluated for catalytic activity in asymmetric carbon-carbon, carbon-heteroatom bond formation reactions. An example of β-amino alcohol ligand in ethylation of benzaldehyde is presented.
 In continuation of our research on the design and synthesis of new chiral ligands for asymmetric catalysis, a new and flexible procedure for the preparation of cis- and trans-bicyclic functionalized chiral azolinium salts, precursors toN-heterocyclic carbenes, derived from L-proline and (S)-pyroglutamic acid has been developed. The efficiency of these NHC ligands has been evaluatedin asymmetric allylic substitution and in conjugate addition of Grignard reagent to α,β-unsaturated ketones. This work is currently underway in our group
In continuation of our research on the design and synthesis of new chiral ligands for asymmetric catalysis, a new and flexible procedure for the preparation of cis- and trans-bicyclic functionalized chiral azolinium salts, precursors toN-heterocyclic carbenes, derived from L-proline and (S)-pyroglutamic acid has been developed. The efficiency of these NHC ligands has been evaluatedin asymmetric allylic substitution and in conjugate addition of Grignard reagent to α,β-unsaturated ketones. This work is currently underway in our group
![]()
G. Vo-Thanh, Prof; C. Bournaud, MCF; M. Toffano, CR CNRS; A. Thomasset, L.Bouchardy, PhD students
Reversible (chiral) ionic liquids: Design, synthesis and applications in (asymmetric) organic synthesis
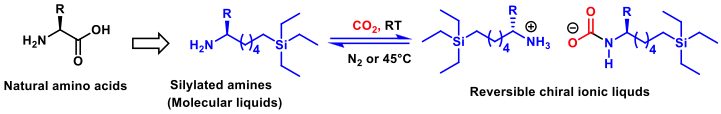
In continuation of our research on the synthesis and applications of chiral ionic liquids as new chiral reaction media for asymmetric synthesis and catalysis, of which G. Vo-Thanh is one of the pioneers in this research field,we have managed to synthesize and formulate, in collaboration with Dr. F.-D. Boyer (ICSN-Gif sur Yvette), a novel class of reversible chiral ammonium carbamate-based ionic liquids derived from natural amino acids. Silylamines reacted reversibly with CO2 to form ammonium carbamates referring to as RevCILs. Some physical properties of these chiral ammonium carbamate salts were measured, especially their reversibility temperature and their stability which were determined thanks to DSC and TGA analyses. The properties of reversibility, chirality, and ease of preparation should make these silylamine-ionic liquid phases as promising solvents and/or catalysts for several applications, especially in asymmetric catalysis fields. It should be noted that for the first time, single-component RevCILS derived from natural amino acids have been reported. This work is currently underway in our group.
![]()
G. Vo-Thanh, Prof; C. Bournaud, MCF; M. Toffano, CR CNRS; V. Rodriguez-Ruiz, Post-doc, L. Bouchardy PhD student
Fluorinated compounds chemistry
This work focuses on the preparation of a new family of (chiral) perfluorinated sulfoximines and sulfinimines. Different transformations and functionalizations of fluorinated sulfoximines have been developed in collaboration with Dr. E. Magnier’s group in Versailles. These new compounds are used as organocatalysts or (chiral) ligands for transition metal catalyzed reactions. This methodology is applied to the synthesis of therapeutic products, eg Prazosin analogues.
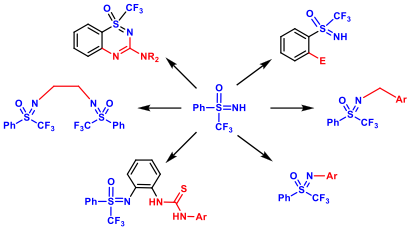
![]()
G. Vo-Thanh, Prof; C. Bournaud, MCF; M. Toffano, CR CNRS; T.–N. Le, PhD student, Vietnamese Government Scholarships-Program ‘Chimie Pharmaceutique
Synthesis of Bio-based polymers for antibacterial surface applications. Structure-Antibacterial Activity Relationships (SAAR) studies
As part of an agreement signed between Paris-Sud University and VAST (Vietnam Academy of Science and Technology), G. Vo-Thanh, in collaboration with researchers at the Institute of Chemistry of Natural Substances in Hanoi, has developed the synthesis of Febrifugine and GA derivatives. Thus, 10 Febrigugine and 25 GA derivatives were synthesized. In vitro, antimalarial activity evaluation, for Febrifugine derivatives, indicated that all synthesized compounds had strong antimalarial activity against both chloroquine-sensitive and -resistant strains of P. falciparum. The cytotoxicity of GA derivatives was tested on the cancerous cell lines Lu1, HepG2, RD and showed very promising results. SAR studies are currently underway on new synthesized analogs.
![]()
G. Vo-Thanh, Prof; C. Bournaud, MCF; M. Toffano, CR CNRS; N. Marets, Post-doctoral researcher
Structure-Activity Relationship studies of Febrifugine, antimalarial activity, and Gambogic Acid (GA), antitumor activity
Preparation of new polymers and polymeric surfaces with antibacterial properties would be of great interest in different areas such as household, industry, hospital. These polymers could be used respectively as disinfection solution or to manufacture sheets and clothes for protection against nosocomial infections. They could be obtained by a polymerization process using monomers which are obtained in some steps starting from isosorbide. This project, in collaboration with Prof. P. Roger (ICMMO), is funded by the Roquette Company and will not be detailed for confidentiality reasons

![]()
G. Vo-Thanh, Prof.
Enzymatic promiscuity
Recently, M. Toffano, in collaboration with Prof. L. Zouioueche (Annaba University), have developed a simple and eco-friendly preparation of α-aminophosphonates and Bis(α-aminophosphonates) viaa multicomponent Kabachnik-Fields reaction in the presence of an immobilized Candida Antarctica lipase as catalyst under solvent-free conditions
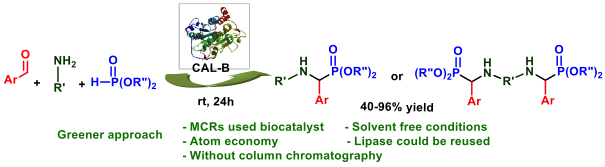
![]()
M. Toffano, CR CNRS
Recent publications:
Vinylogous and stereoselective domino synthesis of pyrano[2,3-c]pyrroles from alkylidene meldrum’s acids. M. Savchuk, G. Vo-Thanh, S. Oudeyer, H. Beucher, J.-F. Brière, Organic & Biomolecular Chemistry, 2024, 22, 2948-2952
Enantioselective Acyl-Transfer/Protonation Reactions with Designed Chiral Thiourea-Iminophosphorane Catalysts. J. Kong, C. Lacroix, C. Bournaud, Y. Yamashita, S. Kobayashi, G. Vo-Thanh, Adv. Synth. Cata, 2024, 366, 1101-1106
Fatty and aromatic acids as acyl donors in enzymatic kinetic resolution of phenylethanol and 1-phenylpropan-2-ol. F. Z. Smaine, M. Merabet-Khelassi, S. Zeror, E. Kolodziej, M. Toffano, L. Aribi-Zouioueche, Monatshefte für Chemie - Chemical Monthly, 2024
2-Hydroxymethyl-18-crown-6 as an efficient organocatalyst for a-aminophosphonates synthesized under eco-friendly conditions, DFT, molecular docking and ADME/T studies. S. Guezane-Lakoud, M. Ferrah, M. Merabet-Khelassi, N. Touil, M. Toffano, L. Aribi-Zouioueche, Journal of Biomolecular Structure and Dynamics, 2023, 0739-1102
Full factorial optimization of α-aminophosphonates synthesis using diphenylphosphinic acid as efficient organocatalyst. M. Ferrah, S. Guezane-Lakoud, H. Bendjeffal, R. Aissa, M. Merabet-Khelassi, M. Toffano, L. Aribi-Zouioueche, Reaction Kinetics, Mechanisms and Catalysis, 2023, 136, 165-182
Practical access to (S)-heterocyclic aromatic acetates via CAL-B/Na2CO3-deacylation and Mitsunobu reaction protocol. N. Braïa, M. Merabet-Khelasi, M. Toffano, L. Aribi-Zouioueche, Biocatalysis and Biotransformation, 2023, 41, 261-269
An Organocatalytic and Stereoselective Vinylogous Domino Process towards Thiochromans. P. Milbeo, A. Lebrêne, M. Savchuk, G. Vo-Thanh, S. Oudeyer, H. Beucher, J.-F. Brière, Chemistry—A European Journal, 2023
Electrochemical Synthesis of Hetero[7]helicenes Containing Pyrrole and Furan Rings via an Oxidative Heterocoupling and Dehydrative Cyclization Sequence. M. S. H. Salem, M. I. Khalid, M. Sako, K. Higashida, C. Lacroix, M. Kondo, R. Takishima, T. Taniguchi, M. Miura, G. Vo-Thanh, H. Sasai, S. Takizawa, Adv. Synth. Catal, 2023, 365, 373-380
Vinblastine loaded on graphene quantum dots and its anticancer applications. T. H. Au, B. N. Nguyen, P. H. Nguyen, S. Pethe, G. Vo-Thanh, T. H. Vu Thi, Journal of Microencapsulation, 2022, 39, 239-251
Access to valuable building blocks by regio-and enantioselective ring-opening of itaconic anhydride using lipase catalysis. N. Braia, M. Merabet-Khelassi, M. Toffano, R. Guillot, L. Aribi-Zouioueche, Org. Biomol. Chem., 2022, 20, 2693-2703
New promising generation of phosphates α-aminophosphonates: Design, Synthesis, In-vitro biological evaluation and Computational study. R. Aissa, S. Guezane-Lakoud, L. Gali, M. Toffano, A. Ignaczak, M. Adamiak, M. Merabet-Khelassi, R. Guillot, L. Aribi-Zouioueche, Journal of Molecular Structure, 2022, 1247, 131336
Two Approaches for CAL-B-Catalyzed Enantioselective Deacylation of a Set of α-Phenyl Ethyl Esters: Organic Solvent with Sodium Carbonate and Micro-aqueous Medium. S. Razi, S. Zeror, M. Merabet-Khelassi, E. Kolodziej, M. Toffano, L. Aribi-Zouioueche, Catalysis Letters, 2021, 151, 2603-2611
Fiaud’s Acid, a novel organocatalyst for diastereoselective bis α-aminophosphonates synthesis with in-vitro biological evaluation of antifungal, antioxidant and enzymes inhibition potential. R. Aissa, S. Guezane-Lakoud, M. Toffano, L. Gali, L. Aribi-Zouioueche, Bioorganic & Medicinal Chemistry Letters, 2021, 41, 128000
Bifunctional N-Heterocylic Carbene-Catalyzed Highly Enantioselective Trans-Cyclopentannulation of Enals and Enones via Homoenolate. Z. Jiang, M. Toffano, G. Vo-Thanh, C. Bournaud, ChemCatChem, 2021, 13, 712-717
Enantioselective Hydrophosphonylation of N-Boc Imines using Chiral Guanidine–Thiourea Catalysts. L. Chassillan, Y. Yamashita, W.-J. Yoo, M. Toffano, R. Guillot, S. Kobayashi, G. Vo-Thanh, Org. Biomol. Chem., 2021, 19, 10560-10564
Auto Tandem Catalysis: Asymmetric Vinylogous Cycloaddition / Kinetic Resolution Sequence for the Enantioselective Synthesis of Spiro-Dihydropyranone from Benzylidene Meldrum’s Acid. M. Toffano, R. Guillot, C. Bournaud, J.-F. Brière, G. Vo-Thanh, Advanced Synthesis & Catalysis, 2021, 363, 4452-4458
Structural modification and biological activity studies of Tagitinin C and its derivatives. T. H. Au, C. Skarbek, S. Pethe, R. Labruère, J.-P. Baltaze, T. P. Hoa Nguyen, T. T. H. Vu, G. Vo-Thanh, Tetrahedron, 2021, 92, 132248
Alkylidene Meldrum’s Acids as Novel Platforms for the Vinylogous Synthesis of Dihydropyranones. G. Vo-Thanh, S. Wittmann, T. Martzel, C.-T. Pham Truong, M. Toffano, S. Oudeyer, R. Guillot, C. Bournaud, V. Gandon, J.-F. Brière, Angewandte Chemie International Edition, 2021, 60, 11110-11114
Using solid carriers impregnated with ammonium ionic liquids for platinum(IV) recovery from chloride solutions. T. L. T. Bui, T. N. H. Uong, K. D. Nguyen, T. K. D. Hoang, G. Vo-Thanh, Korean Journal of Chemical Engineering, 2020, 37, 2262-2272
Vanadium(V) Complex-Catalyzed One-Pot Synthesis of Phenanthridines via a Pictet-Spengler Dehydrogenative Aromatization Sequence. M. Sako, R. Losa, T. Takiishi, G. Vo-Thanh, S. Takizawa, H. Sasai, Catalysts, 2020, 10, 860
Dialkyl Imidazolium Acetate Ionosilica as efficient and Recyclable Organocatalyst for Cyanosilylation Reactions of Ketone. T. D. Tran, A. D. Rodrigues, G. Vo-Thanh, P. Peter Hesemann, Green Energy & Environment, 2020, 5, 130-137
Synergetic catalysis for one-pot bis-alkoxycarbonylation of terminal alkynes over Pd/Xantphos-Al(OTf)3 bi-functional catalytic system. W.-D. Guo, L. Liu, S.-Q. Yang, X.-C. Chen, Y. Lu, G. Vo-Thanh, Y. Liu, ChemCatChem, 2020, 12, 1376-1384
Novel One-Pot Access to Diastereoisomeric Tertiary Phospholanes Oxides by Using Enantiomerically Pure Phospholane Oxides Under Catalyst-Free Conditions. S. G. Lakoud, R. Aissa, R. Guillot, M. Toffano, L. Aribi-Zouioueche, ChemistrySelect, 2020, 5, 379-383
Chiral catalysts derived from biomass: design, synthesis and applications in asymmetric catalysis. T. T. D. Ngo, K.-D. Huynh, H. Ibrahim, T. H. Nguyen, C. Bournaud, M. Toffano, G. Vo-Thanh, Vietnam Journal of Chem, 2019, 57, 670-680
Diastereoselective synthesis of novel bis(α-aminophosphonates) by lipase catalytic promiscuity. R. Aissa, S. Guezane Lakoud, E. Kolodziej, M. Toffano, L. Aribi-Zouioueche, New. J. Chem., 2019, 43, 8153-8159
Photoredox-initiated 1,2-Difunctionalization of Alkenes with N-Chloro S-Fluoroalkyl Sulfoximines. A. Prieto, P. Diter, M. Toffano, J. Hannedouche, E. Magnier, Adv. Synth. Catal., 2019, 361, 436-440
Divergent Synthesis of 1,2-Benzo[e]thiazine and Benzo[d]thiazole Analogues Containing a S-Trifluoromethyl Sulfoximine Group: Preparation and New Properties of the Adachi Reagent. A.-L. Barthelemy, E. Anselmi, T.-N. Le, G. Vo-Thanh, R. Guillot, K. Miqueu, E. Magnier, JOURNAL OF ORGANIC CHEMISTRY, 2019, 84, 4086-4094
Facile Preparation of Vinyl S-Trifluoromethyl NH Aryl Sulfoximines. A.-L. Barthelemy, A. Prieto, P. Diter, J. Hannedouche, M. Toffano, E. Anselmi, E. Magnier, Eur. J. Org. Chem., 2018, 3764-3770
An efficient and recyclable ionic diphosphine-based Ir-catalyst for hydroaminomethylation of olefins with H2O as hydrogen source. H. Liu, D. Yang, D.-L. Wang, P. Wang, Y. Lu, G. Vo-Thanh, Y. Liu, Chemical Communications, 2018, 54, 7979-7982
Novel Class of Reversible Chiral Ionic Liquids Derived from Natural Amino Acids: Synthesis and Characterization. L. Bouchardy, V. Rodriguez-Ruiz, F.-D. Boyer, C. Bournaud, M. Toffano, P. Judeinstein, G. Vo-Thanh, ChemistrySelect, 2018, 3, 958-962
Amphiphilic Zwitterionic phosphine based Au(I)-complex as efficient and recyclable catalyst for hydration of alkynes free of additional additives. X. Chen, X. Ye, W.-Y. Liang, Q. Zhou, G. Vo-Thanh, Y. Liu, Molecular Catalysis, 2018, 448, 171-176
Imidazolium based Ionic liquids as efficient reagents for lignin C-O bond cleavage. M. Thierry, A. Majira, B. Pégot, L. Cezard, F. Bourdreux, G. Clément, F. Perreau, S. Boutet-Mercey, P. Diter, G. Vo-Thanh, C. Lapierre, P.-H. Ducrot, E. Magnier, S. Baumberger, B. Cottyn, ChemSusChem, 2018, 11, 439-448
Promiscuous lipase catalyzed a new P–C bond formation: Green and efficient protocol for one‐pot synthesis of α‐aminophosphonates. S. Guezane‐lakoud, M. Toffano, L. Aribi‐zouioueche, Heteroatom Chemistry, 2017, 28, e21408
Fast and Efficient Hantzsch Synthesis using Acid-activated and Cation-exchanged Montmorillonite Catalysts under Solvent-free Microwave Irradiation Conditions. D. D. Pham, N. T. Le, G. Vo-Thanh, ChemistrySelect, 2017, 2, 12041-12045
Chiral Organocatalyzed Intermolecular Rauhut–Currier Reaction of Nitroalkenes with Ethyl Allenoate. S. Takizawa, M. Sako, K. Kishi, M. Shigenobu, G. Vo-Thanh, H. Sasai, Chemical and Pharmaceutical Bulletin, 2017, 65, 997-999
Efficient and Green Synthesis of 4H-pyran derivatives under Ultrasound Irradiation in the presence of K2CO3 supported on Acidic Montmorillonite. D. D. Pham, G. Vo-Thanh, N. T. Le, Synthetic Communications, 2017, 47, 1684-1691
S-Trifluoromethyl Sulfoximine as a Directing Group in Ortho-Lithiation Reaction toward Structural Complexity. T.-N. Le, P. Diter, B. Pégot, C. Bournaud, M. Toffano, R. Guillot, G. Vo-Thanh, E. Magnier, Org. Lett., 2016, 18, 5102-5105
Organocatalyzed [4+2] Annulation of All-Carbon Tetrasubstituted Alkenes with Allenoates: Synthesis of Highly Functionalized 2H- and 4H-Pyran Derivatives. T.-T.-D. Ngo, K. Kishi, M. Sako, M. Shigenobu, C. Bournaud, M. Toffano, R. Guillot, J.-P. Baltaze, S. Takizawa, H. Sasai, G. Vo-Thanh, ChemistrySelect, 2016, 1, 5414-5420
Biosourced Ligands from Isosorbide for the Ethylation of Aldehydes or Alkynylation of Imines. K.-D. Huynh, H. Ibrahim, L. Bouchardy, C. Bournaud, E. Kolodziej, M. Toffano, G. Vo-Thanh, Asian Journal of Organic Chemistry, 2016, 1242-1246
Phosphine–Thiourea-Organocatalyzed Asymmetric C−N and C−S Bond Formation Reactions. T.-T.-D. Ngo, T.-H. Nguyen, C. Bournaud, R. Guillot, M. Toffano, G. Vo-Thanh, Asian Journal of Organic Chemistry, 2016, Volume 5, Issue 7, 895–899
Synthesis of Novel Triazolo Cyclobutane Nucleoside Analogues. T.-T.-T. Tran, N.-T. Ngo, T.-H. Dinh, G. Vo-Thanh, S. Legoupy, Bull. Korean Chem. Soc, 2015, 36, 1390-1395
Functionalized S-perfluorinated sulfoximines: preparation and evaluation in catalytic process. T.-N. Le, P. Diter, B. Pégot, C. Bournaud, M. Toffano, R. Guillot, G. Vo-Thanh, Y. Yagupolskii, E. Magnier, Journal of Florine Chemistry, 2015, 179, 179-187
P-Aryl-Diphenylphospholanes and their Phospholanium Salts as Efficient Monodentate Ligands for Asymmetric Rhodium-Catalyzed Hydrogenation. C. Dobrota, J.-C. Fiaud, M. Toffano, ChemCatChem, 2015, 7, 144-148

