Homogeneous Organometallic Catalysis Group
The research activity of our group focuses on the development of structurally-defined organometallic complexes from earth-abundant metals (Ln, Mn, Fe, Co…) for atom-economical catalytic applications (hydro- and di-functionalisation, dehydrocoupling…) targeting key and useful synthetic building blocks. Our recent findings with well-defined low-coordinate first-row late transitions metal (Fe, Co) and rare-earth (Sc, Y, Sm, Yb) offer opportunities to explore and develop novel and efficient catalytic reactivities for the (stereo)selective formation of carbon-carbon, carbon-heteroatom bonds or other types of bonds from ubiquitous starting materials. To do so, we will focus our efforts towards a better understanding of the parameters affecting the reactivity and the selectivity of our designed complexes, at different metal oxidation states, in elemental reactions of organometallic chemistry as key steps in many catalytic cycles. In parallel to these studies on atom-economical reactions, part of research is also devoted to the development of asymmetric step-economical catalytic protocols based on one-pot catalysis with a particular focus on asymmetric metallo-organo assisted tandem catalysis. Our group has a mechanistically-driven approach based on the complementarity between experimental and computational studies. This approach is at the core of our research and is carried out in close collaboration with in-house or external (French and international) research teams, mainly for theoretical studies.
Alkene Hydroamination
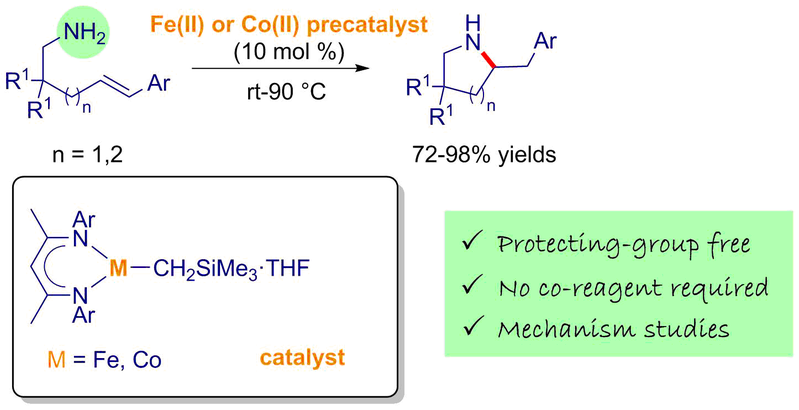
As part of a research program devoted to the development of well-defined and low-coordinate first-row transition metal complexes for atom-economical catalytic applications, we have synthesized and characterized a novel family of structurally-defined β-diketiminato-iron(II) and -cobalt(II) alkyl complexes. These complexes represent first examples of the Fe(II)- and Co(II)-mediated hydroamination of electronically unbiased primary amines by displaying unique catalytic abilities of promoting the selective exo-cyclohydroamination of primary aliphatic alkenylamines. For both catalytic reactions, we have undertaken comprehensive mechanistic investigations by means of complementary experimental (deuterium-labelling, kinetic and stoichiometric experiments) and computational studies. The computational investigations for iron and cobalt systems have been done in collaboration with Dr S. Tobisch (University of St Andrews) and Pr A. Llédos/Dr. G. Ujaque (Universitat Autònoma de Barcelona) respectively. On the basis of these collaborative studies, the reaction catalyzed by β-diketiminatoiron(II) complexes favours a stepwise-σ-insertive mechanism over a proton-assisted concerted N–C/C–H bond-forming non-insertive pathway while that catalyzed by β-diketiminatocobalt(II) complexes operates through a stepwise non-insertive mechanism as original alternative to the classically reported hydroamination mechanisms. The deeper knowledge of mechanistic intricacies was exploited further to improve the selectivity and catalytic efficiency of our iron-based systems.
Alkene Hydroalkoxylation
Cyclisation of unactivated alkenols by hydroalkoxylation is one way to produce in one step various cyclic ethers In order to activate the substrates; this transformation requires very efficient catalysts. Rare earths triflates were tested because of their strong Lewis acid property. These complexes were compared in hydroalkoxylation with alkyl rare earths which possess also basic properties. Among various rare earths triflates, it was found that the more reactive is the scandium triflate. The hydrogen atom of the alcohol function would be made more acidic and the mechanism would go through a cationic intermediary. The product is then obtained according a Markovnikov selectivity. Trialky scandium or yttrium, prepared from LiCH2TMS and ReCl3, can be also employed in hydroalkoxylation; in this case the catalyst is transformed with the alkenol into rare earth trialkenolates. In a second step, this insertion of the C=C bond into the O-metal bond can occurred leading also to a Markovnikov product. Both kinds of catalyst are efficient with some differences of activities according the starting materials. Moreover, one chiral complex of alkyl yttrium bearing a binaphtholate derivative gave encouraging results in terms of enantioselectivity.
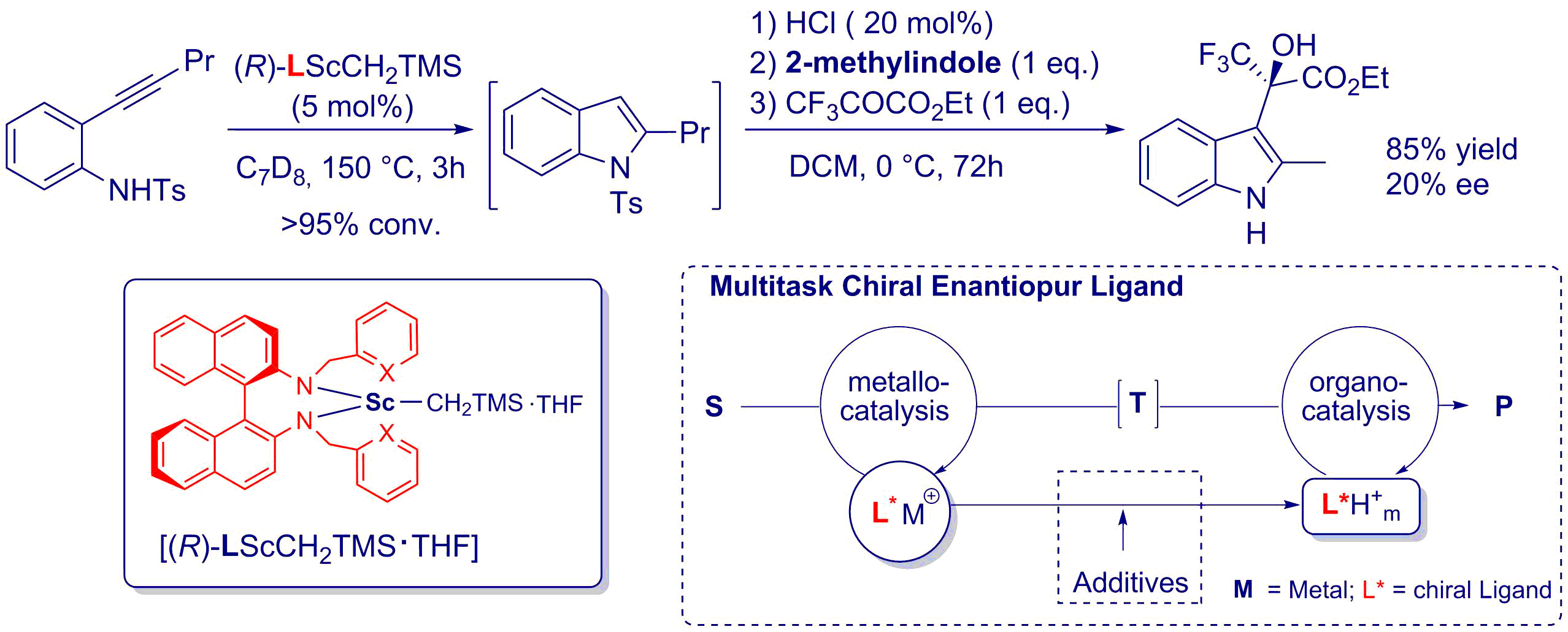
As part of a research program devoted to the development of asymmetric applications of assisted tandem catalysis, we have recently reported the first use of a multitask chiral ligand in asymmetric metallo-organo assisted tandem catalysis. In this protocol, the chiral ligand of the newly designed rare-earth catalyst of the first reaction was converted into a novel organocatalyst (R)-HL·HCl for the second transformation in a tandem sequence alkyne hydroamination / enantio-selective Friedel–Crafts alkylation. The transformation of the organometallic catalyst into the organic catalyst was simply triggered by the addition of HCl and was undoubtedly confirms by heteronuclear 1H–15N NMR correlations through a series of HMBC experiments. Additionally, the observation of some enantioinduction in the tandem sequence confirms the relay of the chiral tetradentate ligand between the two catalysts. This project is done in collaboration with Pr D. Prim (ILV, Université de Versailles).
Recent Publications
A Rationally Designed Iron(II) Catalyst for C(sp3)−C(sp2) and C(sp3)−C(sp3) Suzuki−Miyaura Cross-Coupling. D. Chen, C. Lepori, R. Guillot, R. Gil, S. Bezzenine, J. Hannedouche, Angew. Chem. Int. Ed., 2024, e202408419
Iron-Catalyzed Positional and Geometrical Isomerization of Alkenes. A.-H. Obeid, J. Hannedouche, Adv. Synth. Catal., 2023, 365, 1100-1111
Le fer, un métal « précieux » au service d’une réaction à économie d’atomes. A. Stadler, R. Gil, S. Bezzenine, J. Hannedouche, L'Act. Chim., 2022, 473-474, 33-39
Substrate-Dependent Selectivity in Sc(OTf)3-Catalyzed Cyclization of Alkenoic Acids and N-Protected Alkenamides. H. Zhang, R. Carlino, R. Guillot, R. Gil, S. Bezzenine, J. Hannedouche, Catalysts, 2022, 12, 1481
Visible Light Initiated Palladium-catalyzed Cross-couplings by PPh3 Uncaging from Azobenzene Ruthenium-Arene Complex. L. Rocard, J. Hannedouche, N. Bogliotti, Chem. Eur. J., 2022, 28, e202200519
Earth-Abundant 3d Transition Metal Catalysts for Hydroalkoxylation and Hydroamination of Unactivated Alkenes. L. Rocard, D. Chen, A. Stadler, H. Zhang, R. Gil, S. Bezzenine, J. Hannedouche, Catalysts, 2021, 11, 674-710
Alkene Hydroamination via Earth-Abundant Transition Metal (Fe, Co, Cu and Zn) Catalysis: A Mechanistic Overview. P. Colonna, S. Bezzenine, R. Gil, J. Hannedouche, Adv. Synth. Catal, 2020, 362, 1550-1563
Photoredox-initiated 1,2-Difunctionalization of Alkenes with N-Chloro S-Fluoroalkyl Sulfoximines. A. Prieto, P. Diter, M. Toffano, J. Hannedouche, E. Magnier, Adv. Synth. Catal., 2019, 361, 436-440
C1-symmetric β-Diketiminatoiron(II) Complexes for Hydroamination of Primary Alkenylamines. C. Lepori, R. Guillot, J. Hannedouche, Advanced Synthesis & Catalysis, 2019, 361, 714-719
Experimental and computational mechanistic studies of the β-diketiminatoiron(II)-catalysed hydroamination of alkenes tethered to primary amines. C. Lepori, E. Bernoud, R. Guillot, S. Tobisch, J. Hannedouche, Chem. Eur. J., 2019, 25, 835-844
Facile Preparation of Vinyl S-Trifluoromethyl NH Aryl Sulfoximines. A.-L. Barthelemy, A. Prieto, P. Diter, J. Hannedouche, M. Toffano, E. Anselmi, E. Magnier, Eur. J. Org. Chem., 2018, 3764-3770
Well-Defined β-Diketiminatocobalt(II) Complexes for Alkene Cyclohydroamination of Primary Amines. C. Lepori, P. Gómez-Orellana, A. Ouharzoune, R. Guillot, A. Lledós, G. Ujaque, J. Hannedouche, ACS Catal., 2018, 8, 4446-4451
Hydroamination and Hydroaminoalkylation of Alkenes by Group 3–5 Elements: Recent Developments and Comparison with Late Transition Metals. J. Hannedouche, E. Schulz, Organometallics, 2018, 37, 4313-4326
Mechanistic Insights into First-row Late Transition Metal-catalysed (formal) Hydroamination of Unactivated Alkenes. J. Hannedouche, CHIMIA, 2018, 72, 635-641
Synthesis, characterisation and application of pyridine-modified chitosan derivatives for the first non-racemic Cu-catalysed Henry reaction. F. J. Goncalves, F. Kamal, A. Gaucher, R. Gil, F. Bourdreux, C. Martineau-Corcos, L. V. A. Gurgel, L. F. Gil, D. Prim, Carbohydrate Polymers, 2018, 181, 1206-1212
First-Row Late Transition Metals for Catalytic (Formal) Hydroamination of Unactivated Alkenes. C. Lepori, J. Hannedouche, Synthesis, 2017, 49, 1158-1167
First-Row Late Transition Metals for Catalytic Alkene Hydrofunctionalisation: Recent Advances in C-N, C-O and C-P Bond Formation. S. Bezzenine-Lafollée, R. Gil, D. Prim, J. Hannedouche, Molecules, 2017, 22, 1901-1929
Lithium-Catalyzed anti-Markovnikov Intermolecular Hydroamination Reactions of Vinylarenes and Simple Secondary Amines. S. Germain, M. Lecoq, E. Schulz, J. Hannedouche, ChemCatChem, 2017, 9, 1749-1753
Novel yttrium and zirconium catalysts featuring reduced Ar-BIANH2 ligands for olefin hydroamination (Ar-BIANH2 = bis-arylaminoacenaphthylene). A. Cimino, F. Moscatelli, F. Ferretti, F. Ragaini, S. Germain, J. Hannedouche, E. Schulz, L. Luconi, A. Rossin, G. Giambastiani, New J. Chem., 2016, 40, 10285-10293
Asymmetric Assisted Tandem Catalysis: Hydroamination followed by Asymmetric Friedel–Crafts Reaction from a Single Chiral N,N,N′,N′-Tetradentate Pyridylmethylamine-Based Ligand. A. Aillerie, V. Rodriguez-Ruiz, R. Carlino, F. Bourdreux, R. Guillot, S. Bezzenine-Lafollée, R. Gil, D. Prim, J. Hannedouche, ChemCatChem, 2016, 8, 2455–2460
Mixing and matching chiral cobalt- and manganese-based calix-salen catalysts for the asymmetric hydrolytic ring opening of epoxides. H. Dandachi, E. Zaborova, E. Kolodziej, O. R.-P. David, J. Hannedouche, M. Mellah, N. Jaber, E. Schulz, Tetrahedron: Asymmetry, 2016, 27, 246-253
Recent advances in metal free- and late transition metal-catalysed hydroamination of unactivated alkenes. E. Bernoud, C. Lepori, M. Mellah, E. Schulz, J. Hannedouche, Catal. Sci. Technol., 2015, 5, 2017-2037
Recent developments in alkene hydro-functionalisation promoted by homogeneous catalysts based on earth abundant elements: formation of C-N, C-O and C-P bond. V. Rodriguez-Ruiz, R. Carlino, S. Bezzenine-Lafollee, R. Gil, D. Prim, E. Schulz, J. Hannedouche, Dalton Trans., 2015, 44, 12029-12059
Anti-Markovnikov Hydroamination of Aromatic Alkenes with Secondary Amines Catalyzed by Easily Accessible Yttrium Complexes. S. Germain, E. Schulz, J. Hannedouche, ChemCatChem, 2014, 6, 2065-2073
Well-Defined Four-Coordinate Iron(II) Complexes For Intramolecular Hydroamination of Primary Aliphatic Alkenylamines. E. Bernoud, P. Oulié, R. Guillot, M. Mellah, J. Hannedouche, Angewandte Chemie International Edition, 2014, 53, 4930-4934

