Heterogenization of (asymmetric) catalysts by formation of non-covalent bonds
Having previously demonstrated the efficient reuse of chiral bis(oxazoline)-based organometallic complexes in various asymmetric reactions through formation of non-covalent interactions with different supports, we have adapted this methodology to the recovery of ruthenium complexes, active catalysts to promote metathesis reactions. This project has been supported by the ANR program CD2I CFlow-OM (PI M. Mauduit, Rennes, 2012-2015) which aimed at developing a process for the industrial processing of vegetable oils. In this context, we studied the immobilization of Ru complexes by reversible π-π or charge-transfer complex interactions, on silica supports modified with trinitrofluorenone or on carbon supports. We have evaluated the performances (activity, selectivity, recycling) of these new supported catalysts in the metathesis reaction of benchmark substrates and also unsaturated esters.
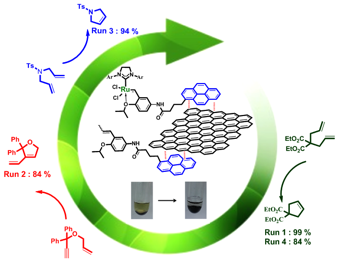
![]()
E. Schulz, DR CNRS, C. Bouvier, IE CNRS, H. Nasrallah, PhD student
Heterogeneization of chiral derivatives of Salen type
In accordance with the principles of green chemistry, we have studied the possibility of recycling chiral organometallic complexes by using them in the form of polymers. Based on our expertise obtained in the field of organic conducting polymers from salen derivatives and their evaluation in catalysis, we have explored heterobimetallic dual catalysis to perform the hydrolytic kinetic resolution (HKR) of different epoxides. In this context, and to favor cooperative activation, we have prepared and tested cobalt-containing Calix-salen complexes, as macromolecular cyclic structures, easily obtained through condensation of appropriate disalicylaldehyde derivatives with enantiopure diamines. Enhanced activity and enantioselectivity have been obtained with these catalysts for the transformation of difficult substrates, such as meso-epoxides, for instance and they have been easily recovered and reused by simple filtration. This last theme is carried out in collaboration with colleagues from the Lebanese University in Beirut thanks to one co-supervised these in the period. It was also supported by the LabEx Charm3At for collaboration with Dr. O. David (ILV, Versailles).
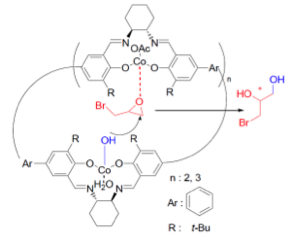
The LabEx also allowed starting two new collaborations, one of them with the polymer group of the SM2B Team (Pr. P. Roger) and Ecole Polytechnique (Dr. A.-C. Gouget) for the synthesis of salen-containing polymers by atom transfer radical polymerization, their characterization and their catalytic properties in HKR. Furthermore, with the group of Dr. V. Huc (ECI), new platforms, based on Calix[8]arenes, have been dedicated to synthesize well defined heterogeneous catalysts that already proved to be efficient for C-C couplings (NHC-Pd complexes) or HKR reactions (Co-Salen complexes).

![]()
Hydroamination reactions with alkali bases, lanthanides or group IV elements
We have amply developed the enantioselective intramolecular hydroamination reaction with chiral lanthanide complexes. This work led us to have many invitations to write reviews in this field. In recent years, we have been keen to discover the reactivity of our complexes to promote the intermolecular version of the reaction. Binaphthylamido alkyl yttrium complexes have thus been proven to promote the anti-Markovnikov addition between various styrene derivatives and secondary amines efficiently. Moreover, we were also able to prepare various β-arylethylamine derivatives by lithium-catalysed anti-Markovnikov selective intermolecular hydroamination reactions of secondary aliphatic amines and vinylarenes. Use of as little as 1.5 mol % LiCH2TMS as solid base in THF proved to be an efficient room-temperature protocol for delivering the targeted products in up to complete conversion. In collaboration with Pr. G. Giambastiani (Florence, Italy), we have shown that cationic complexes of zirconium and hafnium have very competitive catalytic activities with respect to the best zirconium cationic catalysts of the state of the art. Another collaboration in Italy, (Pr. F. Ragaini, Milan) led to the preparation of new biamidoalkyl ytium complexes from bis-arylaminoacenaphthylenes, which proved also to be efficient in the intramolecular hydroamination reaction, specifically for substrates normally reluctant in undergoing cyclization such as those featuring an internal non-activated C=C.

![]()
E. Schulz, DR CNRS, J. Hannedouche, CR CNRS, S. Germain, PhD student
Electrosynthesis and electrocatalysis with Sm(II)
In agreement with the history of our laboratory in the field of lanthanides, we recently developed electrocatalytic procedure based on Sm(II) derivativesas a reducing agent for C-C and C-heteroatom bond forming transformations. The guiding principle of this electrochemical process is based on the facile regeneration of the active low oxidation state catalytic species using a bare samarium electrode as a cathode. We also found that the electrolytic media plays a crucial role in the catalysis and significantly enhanced the reactivity of the SmIIspecies. These results have urged us to evaluate the potential of our catalytic approach to reduce efficiently and selectively other functional groups. Thus, we have recently developed a facile, efficient alternative for synthesizing a wide variety of symmetrical and asymmetrical azobenzenes. Catalytic selective reductions of sulfoxide and phthalimide were demonstrated. Also, in collaboration with Dr C. Gosmini (Ecole Polytechnique) work is ongoing to reduce catalytically CO2 as building block for the synthesis of valuable molecules.
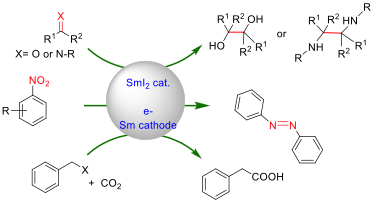
![]()
M. Mellah, MCF, Linhao Sun, Yufeng Zhang and Sakna Bazzi, PhD student, Gaëtan Le Duc Postdoc
Electrochemical Enantioselective Nickel-Catalyzed Cross-Coupling of Aldehydes with Aryl Iodides. L. Hu, J. L. B. L. Fosso, R. Guillot, M. Mellah, E. Schulz, Chemistry – A European Journal, 2024, n/a, e202403432
Electrocatalytic, Sm-Promoted Synthesis of Aminoarenes from Nitroaromatic Derivatives in MeOH. Y.-F. Zhang, E. Schulz, M. Mellah, ChemCatChem, 2024
EurJOC fête ses 25 ans : l’occasion de découvrir le métier d’éditeur. E. Schulz, O. Baslé, J. Crassous, Actualité Chimique, 2023, Juillet-Août, 5-8
Electrogenerated Sm(II)-Catalyzed Carbon Dioxide Reduction for β-Hydrocarboxylation of Styrenes. S. Bazzi, L. Hu, E. Schulz, M. Mellah, Organometallics, 2023, 42, 1425-1431
Direct Quantitative Characterization of Polymer Brushes Obtained by Surface-Initiated ATRP on Silicon. A.-C. Gouget-Laemmel, N. Zidelmal, R. S. B. Soares, N. Barroca-Aubry, D. Dragoe, L. Costa, B. Lepoittevin, H. Salmi-Mani, M. Mellah, C. Henry-De-Villeneuve, F. Ozanam, E. Schulz, P. Roger, ACS Applied Polymer Materials, 2023, 5, 517-528
Post-Modification of Copolymers Obtained by ATRP for an Application in Heterogeneous Asymmetric Salen Catalysis. E. Bakangura, P. Roger, R. S. B. Soares, M. Mellah, N. Barroca-Aubry, A.-C. Gouget-Laemmel, F. Ozanam, L. Costa, J.-P. Baltaze, E. Schulz, Molecules, 2022, 27, 4654
Making Chiral Salen Complexes Work with Organocatalysts. Y.-C. Yuan, M. Mellah, E. Schulz, O. R. P. David, Chem. Rev., 2022, 122, 8841-8883
Samarium(II)-electrocatalyzed chemoselective reductive alkoxylation of phthalimides. Y.-F. Zhang, M. Mellah, Org. Chem.Front., 2022
Synthesis, Catalytic Activity and Comparative Leaching Studies of Calix[8]arene-Supported Pd-NHC Complexes for Suzuki-Miyaura Cross-Couplings. S. Abi Fayssal, T. Naret, J. Buendia, A. Labattut, V. Huc, C. Martini, E. Schulz, Advanced Synthesis & Catalysis, 2022, 364, 947-957
Benzyloxycalix[8]arene supported Pd–NHC cinnamyl complexes for Buchwald–Hartwig C–N cross-couplings. S. Abi Fayssal, T. Naret, V. Huc, J. Buendia, C. Martini, E. Schulz, Catal. Sci. Technol., 2021, 11, 5223-5231
Sulfoxide-Controlled Stereoselective Aza-Piancatelli Reaction. L. Marin, S. Jerhaoui, E. Kolodziej, R. Guillot, V. Gandon, F. Colobert, E. Schulz, J. Wencel-Delord, D. Leboeuf, Advanced Synthesis & Catalysis, 2021, 363, 4277-4282
Chiral Cobalt-Salen Complexes: Ubiquitous Species in Asymmetric Catalysis. E. Schulz, The Chemical Record, 2021, 21, 427-439
Enantiopure isothiourea@carbon-based support: stacking interactions for recycling a lewis base in asymmetric catalysis. Y.-C. Yuan, M. Abd El Sater, M. Mellah, N. Jaber, O. R. P. David, E. Schulz, Org. Chem. Front., 2021, 8, 4699
Chiral Chromium Salen@rGO as Multipurpose and Recyclable Heterogeneous Catalyst. M. Abd El Sater, M. Mellah, D. Dragoe, E. Kolodziej, N. Jaber, E. Schulz, Chemistry – A European Journal, 2021, 27, 9454-9460
Palladium PEPPSI-IPr Complex Supported on a Calix[8]arene: A New Catalyst for Efficient Suzuki–Miyaura Coupling of Aryl Chlorides. A. Labattut, I. Abdellah, J. Buendia, S. Abi Fayssal, E. Adhel, D. Dragoe, C. Martini, E. Schulz, V. Huc, Catalysts, 2020, 10, 1081
Chiral salen complexes in polymeric main-chains for heterogeneous asymmetric catalysis – a brief account. H. Dandachi, X. Hong, F. Ibrahim, H. Nasrallah, A. Zulauf, N. Jaber, M. Mellah, E. Schulz, Vietnam Journal of Chemistry, 2020, 58, 29-39
Calixarene-supported Pd–NHC complexes as efficient catalysts for scalable Suzuki–Miyaura cross–couplings. A. Labattut, S. Abi Fayssal, J. Buendia, I. Abdellah, V. Huc, C. Martini, E. Schulz, React. Chem. Eng., 2020, 5, 1509-1514
Aza-Piancatelli Cyclization as a Platform for the Preparation of Scaffolds of Natural Compounds: Application to the Total Synthesis of Bruceolline D. L. Marin, G. Force, V. Gandon, E. Schulz, D. Lebœuf, European Journal of Organic Chemistry, 2020, 5323-5328
Ajelis et Novecal – Des molécules-cages aux fibres extractantes et à la catalyse. E. Schulz, E. Shilova, P. Viel, G. Gros, C. Martini, I. Abdellah, V. Huc, Actualité Chimique, 2019, mars-avril, 30 – 39
Electrogenerated Sm(II)-Catalyzed CO2 Activation for Carboxylation of Benzyl Halides. S. Bazzi, E. Schulz, M. Mellah, Org. Lett., 2019, 21, 10033-10037
CO2 activation by electrogenerated divalent samarium for aryl halide carboxylation. S. Bazzi, G. Le Duc, E. Schulz, C. Gosmini, M. Mellah, Org. Biomol. Chem., 2019, 17, 8546-8550
Chiral Salen Complexes for Asymmetric Heterogeneous Catalysis: Recent Examples for Recycling and Cooperativity. M. Abd el sater, N. Jaber, E. Schulz, ChemCatChem, 2019, 11, 3662-3687
Photooxygenation of 2-propargylfurans: a path to structurally diverse nitrogen-containing 5-membered rings. L. Marin, G. Force, R. Guillot, V. Gandon, E. Schulz, D. Lebœuf, Chem. Commun., 2019, 55, 5443-5446
Synthesis of Cyclopenta[b]piperazinones via an Azaoxyallyl Cation. B. Baldé, G. Force, L. Marin, R. Guillot, E. Schulz, V. Gandon, D. Lebœuf, Org. Lett., 2018, 20, 7405-7409
Calix[8]arene as New Platform for Cobalt-Salen Complexes Immobilization and Use in Hydrolytic Kinetic Resolution of Epoxides. I. Abdellah, C. Martini, A. Dos santos, D. Dragoe, V. Guérineau, V. Huc, E. Schulz, ChemCatChem, 2018, 10, 4761-4767
Hydroamination and Hydroaminoalkylation of Alkenes by Group 3–5 Elements: Recent Developments and Comparison with Late Transition Metals. J. Hannedouche, E. Schulz, Organometallics, 2018, 37, 4313-4326
Benzyloxycalix[8]arene: a new valuable support for NHC palladium complexes in C–C Suzuki–Miyaura couplings. I. Abdellah, P. Kasongo, A. Labattut, R. Guillot, E. Schulz, C. Martini, V. Huc, Dalton Trans., 2018, 47, 13843-13848
Synthesis, characterization and catalytic properties of salen-containing polymers obtained by atom transfer radical polymerization. N. Zidelmal, N. Barroca-Aubry, B. Lepoittevin, M. Mellah, L. Costa, F. Ozanam, A.-C. Gouget-Laemmel, E. Schulz, P. Roger, Polymer, 2018, 135, 261-270
A diversity-oriented synthesis of cyclopenta[b]pyrroles and related compounds through a calcium(II)/copper(II) catalytic sequence. L. Marin, R. Guillot, V. Gandon, E. Schulz, D. Lebœuf, Org. Chem. Front., 2018, 5, 640-647
Convenient Electrocatalytic Synthesis of Azobenzenes from Nitroaromatic Derivatives Using SmI2. Y.-F. Zhang, M. Mellah, ACS catalysis, 2017, 12, 8480-8486
Lithium-Catalyzed anti-Markovnikov Intermolecular Hydroamination Reactions of Vinylarenes and Simple Secondary Amines. S. Germain, M. Lecoq, E. Schulz, J. Hannedouche, ChemCatChem, 2017, 9, 1749-1753
One-Pot Assembly of Highly Functionalized Cyclopenta[b]pyrroles via a Calcium(II)- and Copper(II)-Catalyzed Reaction Sequence. L. Marin, V. Gandon, E. Schulz, D. Lebœuf, Advanced Synthesis & Catalysis, 2017, 359, 1157-1163
Novel yttrium and zirconium catalysts featuring reduced Ar-BIANH2 ligands for olefin hydroamination (Ar-BIANH2 = bis-arylaminoacenaphthylene). A. Cimino, F. Moscatelli, F. Ferretti, F. Ragaini, S. Germain, J. Hannedouche, E. Schulz, L. Luconi, A. Rossin, G. Giambastiani, New J. Chem., 2016, 40, 10285-10293
Non covalent immobilization of pyrene-tagged ruthenium complexes onto graphene surfaces for recycling in olefin metathesis reactions. H. Nasrallah, S. Germain, P. Queval, C. Bouvier, M. Mauduit, C. Crévisy, E. Schulz, Journal of Molecular Catalysis A: Chemical, 2016, 425, 136-146
Harnessing the Lewis Acidity of HFIP through its Cooperation with a Calcium(II) Salt: Application to the Aza-Piancatelli Reaction. D. Lebœuf, L. Marin, B. Michelet, A. Perez-Luna, R. Guillot, E. Schulz, V. Gandon, Chemistry – A European Journal, 2016, 22, 16165-16171
Mixing and matching chiral cobalt- and manganese-based calix-salen catalysts for the asymmetric hydrolytic ring opening of epoxides. H. Dandachi, E. Zaborova, E. Kolodziej, O. R.-P. David, J. Hannedouche, M. Mellah, N. Jaber, E. Schulz, Tetrahedron: Asymmetry, 2016, 27, 246-253
Direct Immobilization of Ru-Based Catalysts on Silica: Hydrogen Bonds as Non-Covalent Interactions for Recycling in Metathesis Reactions. H. Nasrallah, D. Dragoe, C. Magnier, C. Crévisy, M. Mauduit, E. Schulz, ChemCatChem, 2015, 7, 2493-2500
Organic chemistry: One catalyst, two reactions. News &Views article on the paper by Buchwald et al. E. Schulz, Nature, 2015, 517, 280-281
Recent advances in metal free- and late transition metal-catalysed hydroamination of unactivated alkenes. E. Bernoud, C. Lepori, M. Mellah, E. Schulz, J. Hannedouche, Catal. Sci. Technol., 2015, 5, 2017-2037
Recent developments in alkene hydro-functionalisation promoted by homogeneous catalysts based on earth abundant elements: formation of C-N, C-O and C-P bond. V. Rodriguez-Ruiz, R. Carlino, S. Bezzenine-Lafollee, R. Gil, D. Prim, E. Schulz, J. Hannedouche, Dalton Trans., 2015, 44, 12029-12059

