Chimie Inorganique
Artifical photosynthesis
Axis 1 - PHOTOINDUCED ELECTRON TRANSFER
Led by Ally Aukauloo (ally.aukauloo@universite-paris-saclay.fr)
The input of energy in our biosphere comes from the sun. Nature has developed sophisticated antennae to capture and convert solar energy into a chemical potential. This potential is then used to oxidize water through a four-electron four proton process and the reduced equivalents are ultimately used for the reduction of CO2. Designing and elaborating synthetic systems to replicate the early photophysical events in PSII and coupled to catalysis is the essence of artificial photosynthesis.
Proton-controlled Action of an Imidazole as Electron Relay in a Photoredox Triad. P. Gotico, C. Herrero, S. Protti, A. Quaranta, S. Sheth, R. Fallahpour, R. Farran, Z. Halime, M. Sircoglou, A. Aukauloo, W. Leibl (2022) Photochemical and photobiological Sciences 21, 247
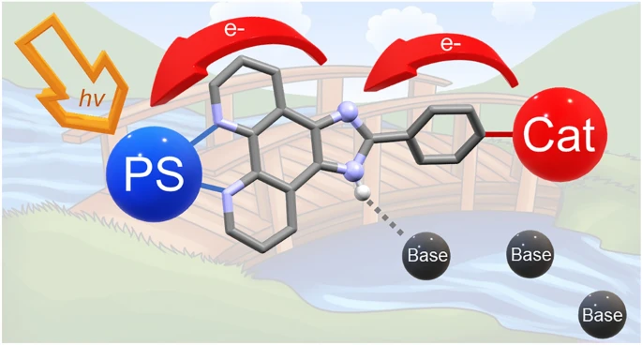
Electron relays play a crucial role for efficient light-induced activation by a photo-redox moiety of catalysts for multi-electronic transformations. Their insertion between the two units reduces detrimental energy transfer quenching while establishing at the same time unidirectional electron flow. This rectifying function allows charge accumulation necessary for catalysis. Mapping these events in photophysical studies is an
important step towards the development of efficient molecular photocatalysts. Three modular complexes comprised of a Ru-chromophore, an imidazole electron relay function, and a terpyridine unit as coordination site for a metal ion were synthesized and the light-induced electron transfer events studied by laser flash photolysis. In all cases, formation of an imidazole radical by internal electron transfer to the oxidized chromophore was observed. The effect of added base evidenced that the reaction sequence depends strongly on the possibility for deprotonation of the imidazole function in a proton-coupled electron transfer process. In the complex with MnII present as a proxy for a catalytic site, a strongly accelerated decay of the imidazole radical together with a decreased rate of back electron transfer from the external electron acceptor to the oxidized complex was observed. This transient formation of an imidazolyl radical is clear evidence for the function of the imidazole group as an electron relay. The implication of the imidazole proton and the external base for the kinetics and energetics of the electron trafficking is discussed.
Phthalocyanine as a Bioinspired Model for Chlorophyll f-Containing Photosystem II Drives Photosynthesis into the Far-Red Region. J. Follana-Berná, R. Farran, W. Leibl, A. Quaranta, Á. Sastre-Santos, A. Aukauloo (2022) Angewandte Chemie International Edition 60, 12284
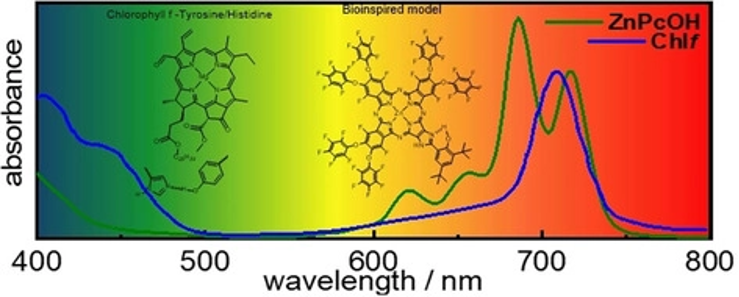
The textbook explanation that P680 pigments are the red limit to drive oxygenic photosynthesis must be reconsidered by the recent discovery that chlorophyll f (Chlf)-containing Photosystem II (PSII) absorbing at 727 nm can drive water oxidation. Two different families of unsymmetrically substituted Zn phthalocyanines (Pc) absorbing in the 700–800 nm spectral window and containing a fused imidazole-phenyl substituent or a fused imidazole-hydroxyphenyl group have been synthetized and
characterized as a bioinspired model of the Chlf/TyrosineZ/Histidine190 cofactors of PSII. Transient absorption studies in the presence of an electron acceptor and irradiating in the far-red region evidenced an intramolecular electron transfer process. Visible and FT-IR signatures indicate the formation of a hydrogen-bonded phenoxyl radical in ZnPc II-OH. This study sets the foundation for the utilization of a broader spectral window for multi-electronic catalytic processes with one of the most robust and efficient dyes.
Enhanced Photoinduced Electron Transfer Through a Tyrosine Relay in a De Novo Designed Protein Scaffold Bearing a Photoredox Unit and a FeIIS4 Site. A. Tebo, A. Quaranta, V. L. Pecoraro, A. Aukauloo (2021) ChemPhotoChem 5 : 665-668
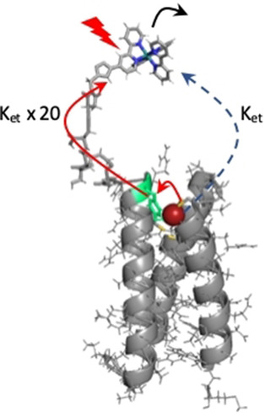
We have designed an electron transfer chain incorporated into a de novo protein scaffold, which is capable of photoinduced intramolecular electron transfer between a photoredox unit and a FeIIS4 site through a tyrosine amino acid relay. The kinetics were characterized by nanosecond laser pulse photolysis and revealed that electron transfer from the photoredox unit [RuIIIbpymal]3+ proceeds most efficiently via a tyrosine located circa 16 Å from Rubpymal ((bpymal=1-((1-([2,2′-bipyridin]-4-yl)-1H-1,2,3-triazol-4-yl)methyl)-1H-pyrrole-2,5-dione)). Removal of the tyrosine as the electron relay station results in a 20-fold decrease in the apparent rate constant for the electron transfer.
Tracking light-induced electron transfer toward O2 in a hybrid photoredox-laccase system. R. Farran, Y. Mekmouche, N. T. Vo, C. Herrero, A. Quaranta, M. Sircoglou, F. Banse, P. Rousselot-Pailley, A. J. Simaan, A. Aukauloo, T. Tron, W. Leibl (2021) iScience 24, 102378
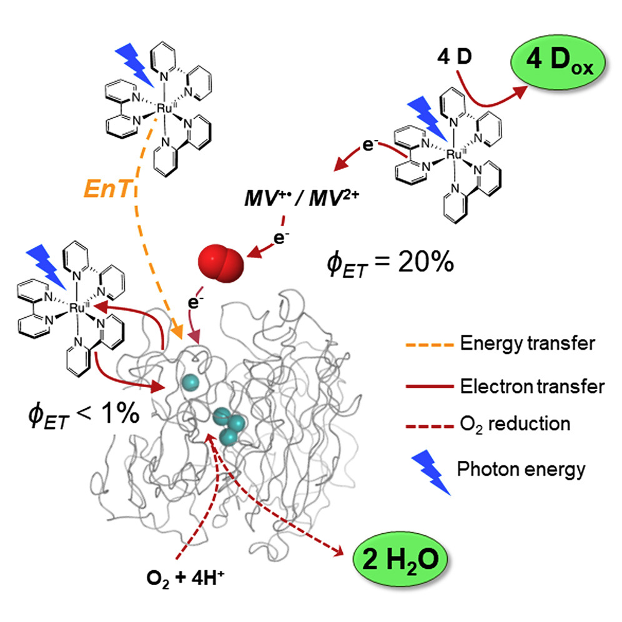
Photobiocatalysis uses light to perform specific chemical transformations in a selective and efficient way. The intention is to couple a photoredox cycle with an enzyme performing multielectronic catalytic activities. Laccase, a robust multicopper oxidase, can be envisioned to use dioxygen as a clean electron sink when coupled to an oxidation photocatalyst. Here, we provide a detailed study of the coupling of a [Ru(bpy)3]2+ photosensitizer to laccase. We demonstrate that efficient laccase reduction requires an electron relay like methyl viologen. In the presence of dioxygen, electrons transiently stored in superoxide ions are scavenged by laccase to form water instead of H2O2. The net result is the photo accumulation of highly oxidizing [Ru(bpy)3]3+. This study provides ground for the use of laccase in tandem with a light-driven oxidative process and O2 as one-electron transfer relay and as four-electron substrate to be a sustainable final electron acceptor in a photocatalytic process.
A TyrZ-His190 model
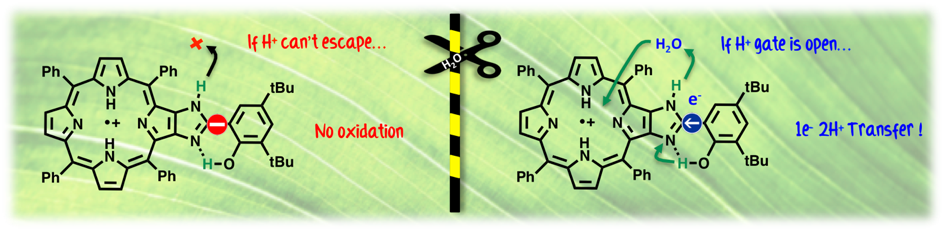
Photosystem II is the enzyme that captures sunlight to oxidize water to O2. If the pathways for electron transfer are quite well understood, the proton transfer pathways are still obscure. Recent advanced X-ray studies of PSII suggest that the TyrosineZ/His190 amino acid pair is also implicated in the expulsion of protons from the Oxygen Evolving Complex (OEC) rather than a mere electron relay between the chlorophyll pigments and the OEC. We show in this study that water molecules are gating a one-electron and two protons shifts from a biomimetic model of TyrosineZ/His190. Our results provide a first model to pertaining the hypothesis that TyrosineZ/His190 may also be the way out for protons from the OEC.
Water Molecules Gating a Photoinduced One Electron Two Protons Transfer in a Tyr/His model of Photosystem II. G. Chararalambidis, S. Das, A. Trapali, A. Quaranta, M. Orio, Z. Halime, P. Fertey, R. Guillot, C. Athanassios, W. Leibl, A. Aukauloo, M. Sircoglou (2018) Angew. Chem. Int. Ed. 57, 9013-9017
2 electrons charge accumulation
Understanding the parameters ruling light induced charge accumulation in synthetic models is undoubtedly a tough subject for chemists. In this study, we have developed a Pump-Pump Probe set up to monitor a charge accumulation of 2 electrons in a photosensitizer-electron acceptor dyad in presence of a sacrificial electron donor. We have been able to decipher almost the complete constructive and deleterious electron pathways leading to a two-electron charge accumulation at high potential ca. 1.1 eV and with a lifetime of 0.2 ms. This study and home built set-up put us on the track to interrogate light triggered multielectron transfer processes and catalysis.
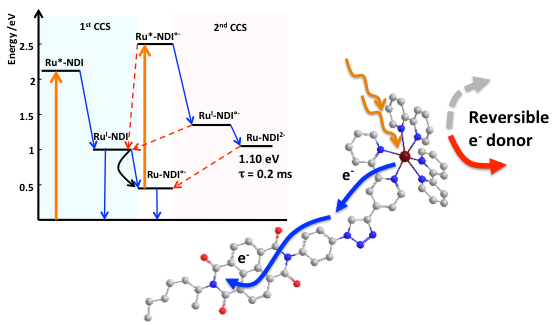
Time-Resolved Interception of Multiple-Charge Accumulation in a Sensitizer–Acceptor Dyad. S. Mendes marinho, M.-H. Ha-Thi, V.-T. Pham, A. Quaranta, T. Pino, C. Lefumeux, T. Chamaillé, W. Leibl, A. Aukauloo (2017) Angew. Chem. Int. Ed. 11, 15936–15940
Time-resolved X-ray absorption spectroscopy, a sophisticated method to track electron accumulation
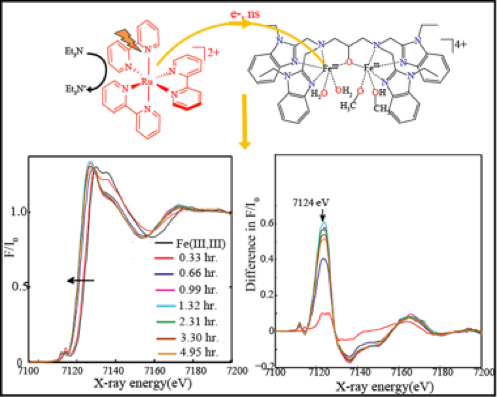
In a recent paper (Angewandte Chemie 2015) we reported on the photocatalytic oxidation of an organic substrate. In collaboration with Dr. D. Moonshiram at the Argonne National Lab USA, we have used time-resolved X-ray absorption spectroscopy in the ns-µs time scale to track the light induced two electron transfer processes in a multi-component photocatalytic system, consisting of [Ru(bpy)3]2+/ a diiron(III,III) model/triethylamine. EXAFS analysis with DFT calculations confirm the structural configurations of the diiron(III,III) and reduced diiron(II,II) states, therefore bringing support for our mechanistic proposal.
Elucidating the light-induced charge accumulation in an artificial analogue of methane monooxygenase enzymes using time-resolved x-ray absorption spectroscopy. D. Moonshiram, A. Picon, Á. Vázquez-Mayagoitia, X. Zhang, M.-F. Tu, P. Garrido-Barros, J.-P. Mahy, F. Avenier, A. Aukauloo (2015) Chem. Commun. 53, 2725-2728
Another challenge
A stubborn issue in the field of light driven multiple electron transfer processes for small molecules transformation (H2O, H2, CO2, CH4, N2…), concerns the use of sacrificial electron donors or acceptors. This constitutes a limiting factor towards the sustainability of these light driven catalysis. Therefore, it is of utmost priority to preclude these ingredients in photocatalytic processes. We are currently addressing this problem in an ongoing Multiplet ANR project. We are embarked with biologist colleagues from Marseille, to use an enzyme, the Laccase, a tetranuclear copper containing enzyme capable to reduce O2 to water as a renewable sink of electrons.
For more details, see the comprehensive list of our publications HERE.
Last update on August 1st 2023

