Chimie Inorganique
ANR Multiplet — 2015-2019
"Multielectronic light driven water activation for oxygen atom transfer reactions under mild conditions"
Coordinator : T. Tron (Aix Marseille Université)
Inorg. Chem. groups involved as partners : Artifical photosynthesis et Fe complexes, bioinspired catalysts for oxidation
1 PhD student, 2 postdocs
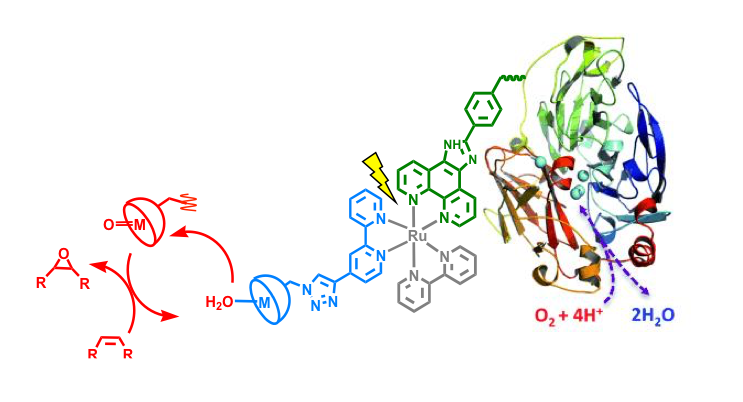
This project is centered on the construction of a self-sufficient system allowing Oxygen Atom Transfer Reactions (OATR) by coupling a synthetic sensitizer-catalyst assembly able to photoactivate H2O and generate highly reactive metal oxo species (2e-,2H+ process) to an active robust multi-copper oxidase (laccase) acting as an electron/proton acceptor that performs O2 reduction (4e-,4H+ process).
Key facts
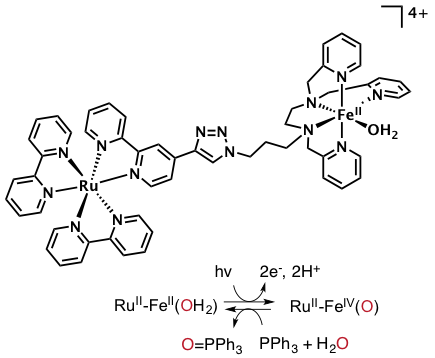
In collaboration with iBiTec-S (CEA), we described the synthesis and study of a RuII–FeII chromophore–catalyst assembly designed to perform the light-induced activation of an iron bound water molecule and subsequent photo-driven oxidation of a substrate. We demonstrate that excitation of the chromophore unit with 450 nm light, in the presence of a sacrificial electron acceptor, triggers a cascade of electron transfers leading to the formation of a high valent iron(IV)–oxo center from an iron(II)-bound water molecule. This proof of concept demonstrate that it is possible to synchronize fast photoinduced electron transfers with slow chemical reactions.
Successive light-induced two electron transfers in a Ru–Fe supramolecular assembly: from Ru–Fe(II)– OH2 to Ru–Fe(IV)–oxo. C. Herrero, A. Quaranta, M. Sircoglou, K. Sénéchal-David, A. Baron, I. M. Marín, C. Buron, J.-P. Baltaze, W. Leibl, A. Aukauloo et al. (2015) Chem. Sci. 6, 2323–2327
In collaboration with our colleagues from Marseille, we have already reported on the proof of concept that it is possible to perform photooxidation of organic substrates in a mixture of a photosensitizer/Laccase without the need for a sacrificial electron donor with O2 acting both as an oxygen atom donor and an electron acceptor
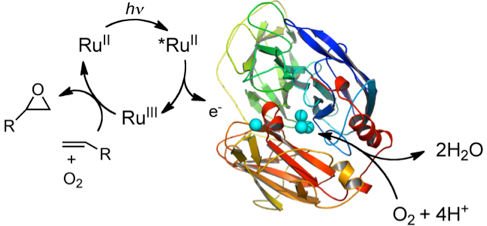
Visible-Light-Driven Oxidation of Organic Substrates with Dioxygen Mediated by a [Ru(bpy)3]2+/Laccase System. L. Schneider, Y. Mekmouche, P. Rousselot-Pailley, A. J. Simaan, V. Robert, M. Réglier, A. Aukauloo, T. Tron (2015) ChemSusChem 8, 3048–3051
However, the mechanism leading to the oxygen atom transfer reaction follows a radical pathway which must be further control for more selective oxidation reaction. We are currently addressing this challenging problem.
Last update on 06.21.2019

