Chimie Inorganique
Fe complexes, bioinspired catalysists for oxidation
Axe 3 — INSERTION F THE COMPLEXES INTO SUPRAMOLECULAR SYSTEMS AND SECOND COORDINATION SPHERE EFFECTS
This project aims at improving the selectivity and efficacy of our catalysts by drawing inspiration from the functioning of metalloenzymes.
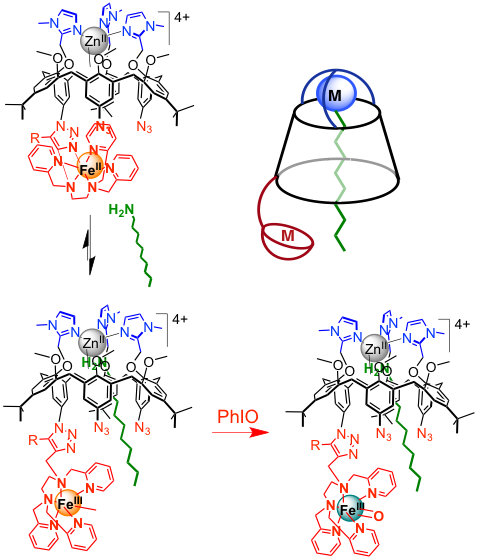
In metalloenzymes, the second coordination sphere in the active site anchors the substrate next to the metal center allowing its efficient and selective transformation. We have developed (collaboration with Pr. O. Reinaud, Univ. Paris Descartes) a new system in which the catalytic unit (Fe complex) is grafted to a calixarene cavity capped by a ZnII complex aimed at binding a substrate and driving it next to the iron center. A detailed coordination chemistry study has revealed how locating at the same time FeII in the amine/pyridine and ZnII in the tris(imidazole)site. The resulting heterodinuclear complex is able to encapsulate linear alkylamines (C7-C12) by binding them at the ZnII. Additionally, stabilization of FeIVO and FeIII(OOH) intermediates at the Fe complex has been demonstrated. Interestingly, the host amine induces a coordination switch of Fe which facilitates the formation of the FeIVO mimicking mimicking the allosteric effect frequently observed in metalloenzymes.
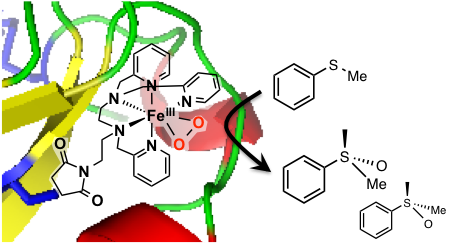
Covalent insertion of a metal complex into a protein is another way to elaborate artificial metalloenzymes. β-lactoglobulin contains one free cysteine which we have targeted for anchoring an FeII complex (Cathymetoxy ANR grant, collaboration with Pr. J.-P. Mahy, ICMMO, Univ. Paris Sud). This biohybrid system catalyzes the sulfoxidation of thioanisole by H2O2 with a 20% ee. A FeIII(OO) intermediate competent for the oxidation of the substrate has been identified.
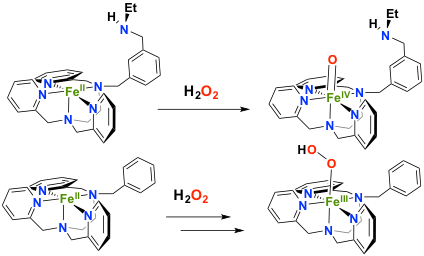
Simpler systems can be prepared by introducing polar groups as second coordination sphere of the metal center in order to pull the electronic density out of the Fe-oxygen intermediates. In the presence of a non coordinating amine as second sphere moiety, the reactivity of an FeII complex with hydrogen peroxide has been drastically changed. In this case, a direct conversion from FeII to FeIVO is observed, whereas a FeIII(OOH) is formed in the absence of this amine (collaboration with Dr F. Avenier, ICMMO, Univ. Paris Sud). This second sphere moiety plays the same role than the histidine residue in the peroxydases active site.
Last update on 11.13.2019

