Amelie Bordage
Build. 670, office 2409 – LCI – ICMMO - UMR 8182
Université Paris-Saclay
Bâtiment 670
17-19 Avenue des Sciences
91400 Orsay
FRANCE
CNRS RESEARCHER (Section 14)
Inorganic chemistry team
"From the molecular switching to nanodevices for electronic" group
Localization : Building 420 (Rue du doyen Georges Poitou) , Ground floor, Office 005

Interests
- Synchrotron radiation based experiments
- Structural and electronic properties of transition metal ions
- Functional molecular materials : Prussian Blue analogues, mixed oxides
- Theoretical calculations of core-level spectroscopies
Experimental techniques
- Core-level spectroscopies : XAS, XES, XMCD
- Synthesis of Prussian Blue analogues (drop-by-drop addition of 2 aqueous solutions)
- Solid synthesis by calcination under oxidizing conditions
- Powder x-ray diffraction (measurements et Rietveld refinement)
Scientific societies/assocations
- SOLEIL Users Organisaion (ORGUES) — Vice-president
- French Association of Synchrotron Radiation Users (AFURS) — Trustee
- International X-ray Absorption Society (IXAS)
- French Chemical Society (SCF)
- French Society of Mineralogy and Cristallgraphy (SFMC) — Trustee
Ongoing projects
1. Development of a new methodology based on transition metal K-edge XMCD for the quantification of small structural distortions
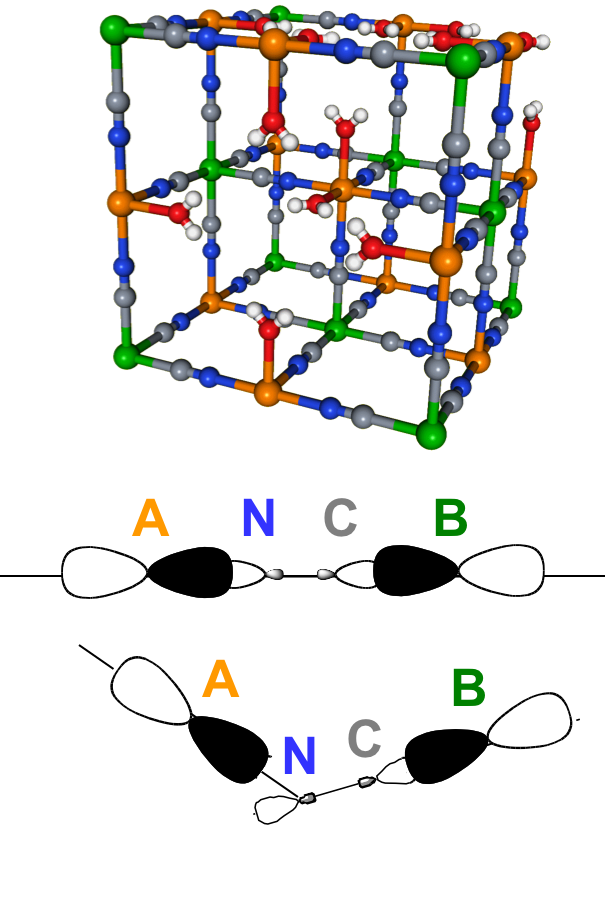
The small structural distortions of the A-NC-B (A,B = transition metals; Figure) linkage seem to control the photomagnetic properties of PBAs. These distortions are however too small for any quantification by classicalt techniques of structural characterization. I am working on a new methodology based on X-ray magnetic circular dichroism (XMCD) at the K-edge of transition metals to quantify these small distortions of the cyanide bridge. This requires the systematic investigation of non-photomagnetic model PBAs
- to establish the quantitative relationship between the XMCD signal and the structural distortions, and
- to disentangle the physical effects originating the Transition metals K-edge XXMCD signals.
When the methodology is established, it will be applied to the investigation of photomagnetic PBAs in order to control their properties and tranfer their working temperature at room temperature.
This project is supported by an Young Researchers ANR (2018-2023) and described in more details here. in more details here.
Techniques : XAS (Transmission and HERFD) and XMCD at the K-edge of transition metals(Fame-UHD @ ESRF, SAMBA, DIFFABS and ODE @ SOLEIL beamlines)
2. Investigation of the photomagnetism of CoFe Prussian Blue analogues
In the yCoFe (X = alkali cation, y = 0-4) PBAs, a pressure-, thermally- and/or photoinduced charge transfer can be observed depending on the stoichiometry. In general, PBAs with 2 alkali cations per unit cell (X2Co4[Fe(CN)6]3.3) always present photomagnetic properties at low temperature, but depending on the alkali cation nature, this photoinduced charge transfer can be successive to a thermally-induced one. For a given alkali cation, the inserted quantity per unit cell adjust this charge transfer : the Cs2CoFe and Cs0.7CoFe PBAs both present a photoinduced charge transfer at low temperature, but in the case of Cs0.7CoFe, a first thermally-induced charge transfer is observed (Figure). Some of these were also successfully synthesized as nanoparticles (~5 nm); in the case of the Rb2CoFe PBA, despite differences in the ground states of each kinfd of particles, the photomagnetic property is retained at the nanoscale. All these previsous studies thus enables to empirically identify the photomagnetic PBA, but oddly these charge-transfer properties are still badly understood.
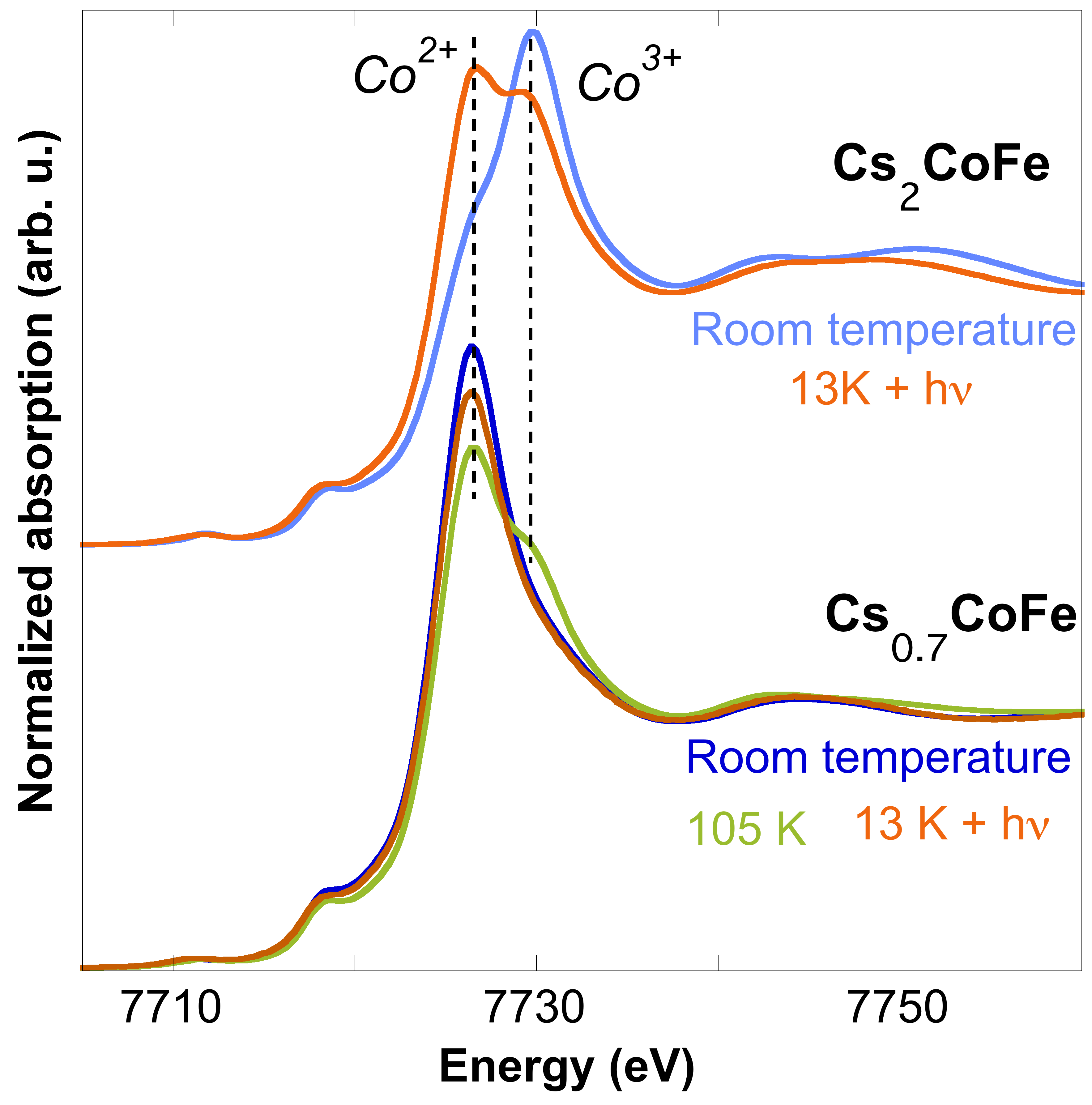
Therefore I lead fundamental investigations of these photomagnetic properties in order to understand :
- the role of the alkali cation in the charge-transfer properties,
- the key-parameters to control photomagnetism and the charge-transfer temperature,
- the effect of the particles size reduction on the photomagnetic properties.
Techniques : XAS (Transmission andHERFD) at the K-edge of transition metals and the the edges of alkali cations (BM20 @ ESRF, SAMBA and LUCIA @ SOLEIL beamlines)
3. Synthesis of mixed oxides from Prussian Blue analogues
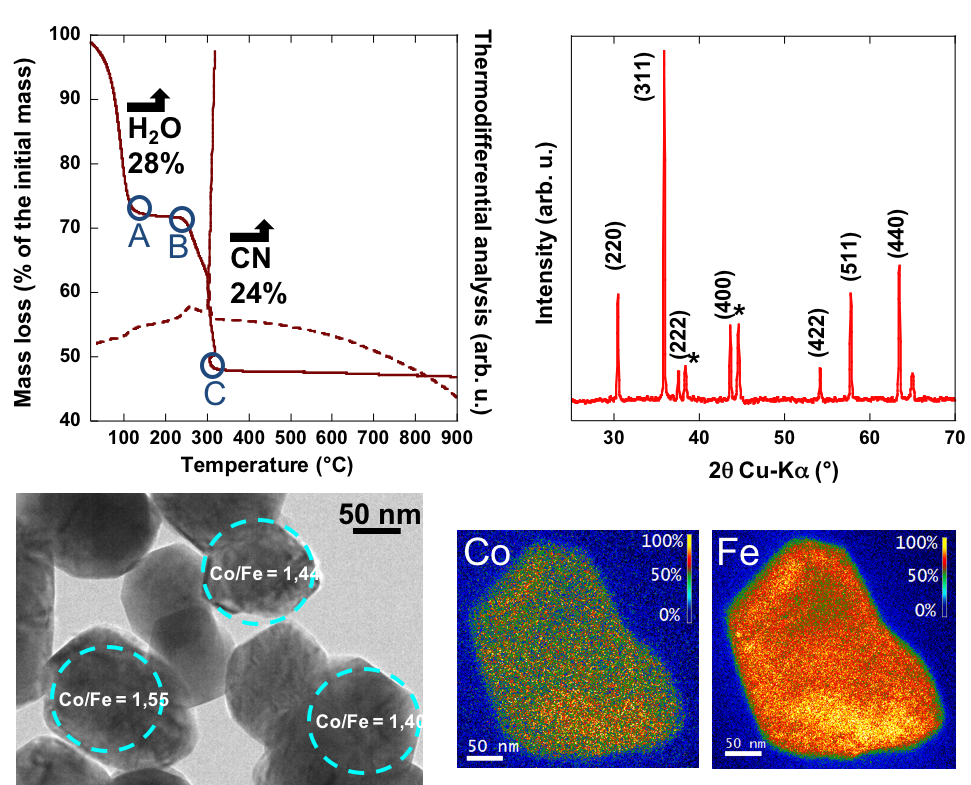
It is well-known that the properties of oxides are controlled by their stoichiometry and synthesis route. A synthesis way reproducible and offering a perfect control of the stoichiometry is so a key-element for the successful development of functional oxides. The calcination in air of PBAs is an appealing route: the PBA stoichiometry is indeed perfectly controlled during by its synthesis, with an homogeneous repartition of the transition metals. The calcination of the CoII4[CoIII(CN)6]2.7 PBA into Co3O4 was first investigated; it lead to the first in situ site-selective XANES experiments, performed to characterize the evolutio of each Co ion type present in the PBA and the Co3O4 spinel. The current studies concentrate on the mixed Co4[Fe(CN)6]2.7 PBA and the formation of the Co1.8Fe1.2O4 spinel (Figure). The different steps of the calcination process were described by TDA/TGA analyses and ex situ powder X-ray diffraction. The synthesis route was then enlarged to the formation of oxides with a controlled stoichiometry between Co1.8Fe1.2O4 and Co3O4 as well as the formation of oxides nanoparticles.
In the framework of this project, my task is to complete the laboratory investigation by in situ XAS measurements of the calcination process in order to finely characterize the oxidation states and local structure of the Fe and Co ions during the calcniation and in the final phase.
Techniques : Powder X-ray diffraction (ex and in situ), in situ XAS at the K-edge of transition metals (FAME @ ESRF et ROCK @ SOLEIL beamlines)
Last publications
A Peculiar Photo-Induced Transformation Exalted in Nanometric Size CoFe Prussian Blue Analogs. G. Balthazar, A. Bordage, G. Fornasieri, L. Altenschmidt, A. Zitolo, A. Bleuzen, ChemPhotoChem, 2023, 7, e202300102
Simple fabrication of e-Fe2O3 nanoparticles containing silica monoliths with enhanced coercitivity. L. Altenschmidt, P. Beaunier, A. Bordage, E. Rivière, G. Fornasieri, A. Bleuzen, ChemNanoMat, 2023, 9, e202200469
Towards quantitative magnetic information from transition metal K-edge XMCD of Prussian Blue analogs. A. N'Diaye, A. Bordage, L. Nataf, F. Baudelet, E. Rivière, A. Bleuzen, Inorganic Chemistry, 2022, 61, 6326-6336
Prussian Blue analogs and transition metal K-edge XMCD: A longstanding friendship. A. Bordage, A. N'Diaye, A. Bleuzen, Comptes Rendus Chimie, 2022, 25, 281-288
Interplay between transition metal K-edge XMCD and magnetism in Prussian Blue analogs. A. N'Diaye, A. Bordage, L. Nataf, F. Baudelet, E. Rivière, A. Bleuzen, ACS Omega, 2022, 7, 36366-36378
Co, Fe and CoFe oxide nanoparticle assemblies within an ordered silica matrix: effects of the metal ions and synthesis pathway on the microstructure and magnetic properties. L. Altenschmidt, P. Beaunier, E. Riviére, G. Fornasieri, A. Bordage, A. Bleuzen, The European Physical Journal Special Topics, 2022
A cookbook for the investigation of coordination polymers by transition metal K-edge XMCD. A. N’Diaye, A. Bordage, L. Nataf, F. Baudelet, T. Moreno, A. Bleuzen, Journal of Synchrotron Radiation, 2021, 28, 1127-1136
Influence of the number of alkali cation on the photo-induced CoIIIFeII-CoIIFeIII charge transfer in CsxCoFe PBAs - A Co K-edgeXANES study. A. Bordage, A. Bleuzen, Radiation Physics and Chemistry, 2020, 175, 108143
(Photo)magnetism in 5 nm nanocrystals of the alkali cation free-CoFe prussian blue analogue embeddedin a silica matrix. A. Bleuzen, M. Goncalves, L. Altenschmidt, G. Fornasieri, A. Bordage, E. Rivière, Chemistry Squared, 2020, 4, 1
Towards the synthesis of mixed oxides with controlled stoichiometry from Prussian blue analogues. V. Trannoy, A. Bordage, J. Dezalay, R. Saint-Martin, E. Rivière, P. Beaunier, C. Baumier, C. La Fontaine, G. Fornasieri, A. Bleuzen, CrystEngComm, 2019, 21, 3634-3643
Transforming a Diamagnetic Ordered Mesoporous Silica Monolith into a Room Temperature Permanent Magnet through Multiscale Control of the Magnetic Properties. V. Trannoy, L. Altenschmidt, G. Fornasieri, A. Bordage, E. Rivière, P. Beaunier, A. Bleuzen, ChemNanoMat, 2018, 4, 1254-1261
Weak Ferromagnetic Interaction at the Surface of the Ferrimagnetic Rb2Co4[Fe(CN)6]3.3·11H2O Photoexcited State. S. Jafri, M.-A. Arrio, A. Bordage, R. Moulin, A. Juhin, C. Cartier Dit Moulin, E. Otero, P. Ohresser, A. Bleuzen, P. Sainctavit, Inorg. Chem., 2018, 57, 7610-7619
Evidence of the Core–Shell Structure of (Photo)magnetic CoFe Prussian Blue Analogue Nanoparticles and Peculiar Behavior of the Surface Species. A. Bordage, R. Moulin, E. Fonda, G. Fornasieri, E. Rivière, A. Bleuzen, J. Am. Chem. Soc., 2018, 140, 10332-10343
Magnetism and Photomagnetism of Prussian Blue Analogue Nanoparticles Embedded in Porous Metal Oxide Ordered Nanostructures. G. Fornasieri, A. Bordage, A. Bleuzen, European Journal of Inorganic Chemistry, 2018, 3-4, 259-271
Effect of S on the aqueous and gaseous transport of Cu in porphyry and epithermal systems: Constraints from in situ XAS measurements up to 600°C and 300bars. M. Louvel, A. Bordage, B. Tripoli, D. Testemale, J.-L. Hazemann, J. Mavrogenes, Chemical Geology, 2017, 466, 500-511
Temperature dependence of X-ray absorption and nuclear magnetic resonance spectra: probing quantum vibrations of light elements in oxides. R. Nemausat, C. Gervais, C. Brouder, N. Trcera, A. Bordage, C. Coelho-Diogo, P. Florian, A. Rakhmatullin, I. Errea, L. Paulatto, M. Lazzeri, D. Cabaret, Phys. Chem. Chem. Phys., 2017, 19, 6246-6256
Ordered Mesoporous Silica Monoliths as a Versatile Platform for the Study of Magnetic and Photomagnetic Prussian Blue Analogue Nanoparticles. R. Moulin, E. Delahaye, A. Bordage, E. Fonda, J.-P. Baltaze, P. Beaunier, E. Rivière, G. Fornasieri, A. Bleuzen, European Journal of Inorganic Chemistry, 2017, 2017, 1303-1313

