High Entropy Oxides
contacts : David Bérardan, Nita Dragoe
high Entropy Oxides are a new class of materials discovered in 2015 by a team from North Carolina State (Rost et al., Nature Communications 6, 8485 (2015)), whose properties have been studied for the first time in our group. When a sufficiently large number of binary oxides are mixed and annealed at high temperature, the main contribution the total Gibbs energy becomes that of the configurational entropy, which leads to the formation of a single phase material that crystallizes in a simple structure, with a homogeneous random distribution of the cations in the lattice, and which can be "frozen" at room temperature by quenching. This is typically the case of the "canonical" composition (MgCoNiCuZn)O, which is obtained by annealing a mixture of the constituting binary oxides at T>875°C, which leads to a new material crystallizing in a simple rocksalt structure with a random distribution of the cations on the cationic sublattice. Therefore, high entropy oxides are not "just" new materials, they constitute a new paradigm in the development of new functional materials, with the configurational entropy that becomes the main parameter.
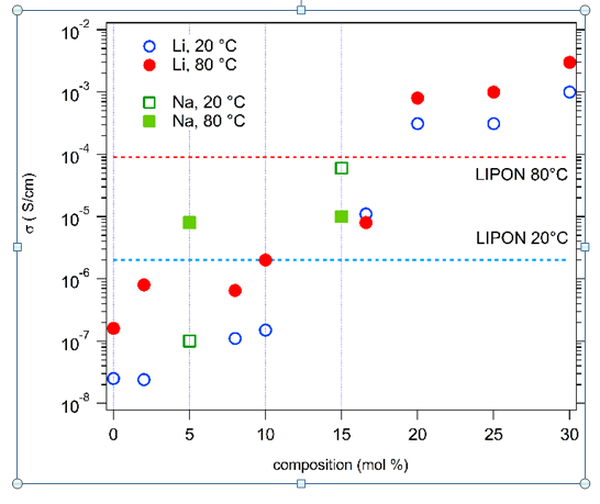
Following the discovery of these new materials, we have started studying their functional properties, as well as testing the various possibilities of substitutions, doping, combinations of cations, (...). Interestingly, we have shown that aliovalent or isovalent substitutions are possible, as well as tuning the stoichiometry of the constituting cations, which greatly increases the number of possible compositions. Regarding the properties, we have shown that some of these compounds have promising dielectric properties, with colossal dielectric constants. Among the various compositions that we have prepared, we have shown the possibility of introducing alcaline elements in the structure. This aliovalent substitution is made possible by a charge compensation, either "external" with a cosubstitution by a +III, +IV or +V cation, either "internal" by the oxidation of Co2+ into Co3+ or the formation of oxygen vacancies. In the later case, the obtained compounds exhibit very promising ionic conductivity values of Li+ or Na+ cations, with for example a superionic conductivity of Li+ larger than 0.1 S/m at room temperature or 0.4 S/m at 80°C (see following figure). These values are more than one order or magnitude larger than that of LiPON, solid state electrolyte used in microbatteries, and of the same order as the best solid state electrolytes known to date, which makes these materials very promising for application as solid state electrolytes in lithium or sodium batteries.
Selected publications:
- "Colossal dielectric constant in high entropy oxides", Physica Status Solidi RRL 10, 328 (2016) (Colossal dielectric constant)
- "Room temperature lithium superionic conductivity in high entropy oxides", Journal of Materials Chemistry A 4, 9536 (2016) (Li+ superionic conductivity and Na+ fast ionic conductivity)

