Méthodologie, Synthèse et Molécules Thérapeutiques
Unsaturated AminoPhosphonates
Our research is focused on the synthesis of aminophosphonates intermediates with high regio- and stereocontrol. Indeed, the phosphonate moiety is a key element in synthetic organic. Associated with an amine it represents an emerging pharmacophore. Despite their wide use as herbicides, insecticides and fungicides, aminophosphonates plays an important role in human life and are used as HIV inhibitors, antitumor agents or antibiotics.
Our work is divided in three parts:
Aminophosphonates Synthesis: Highly functionalized β-amino vinylphosphonates have been prepared from ynamides by a nickel(II)-catalyzed hydrophosphonylation reaction. The adducts are obtanied with high regio and stereoselectivity. Investigations on the mechanism of the reaction is under way to dertermine the scope and limitation of this transformation. (Collaboration Dr. G. Evano, Université de Versailles).
By studying more closely the reactivity of ynamides towards organophosphorus reagents, we have developed a simple and efficient method for the preparation of α-amino allenylphosphonates. First we investigated the chemo and stereoselective reduction of allenylphosphonates to vinylphosphonates. This reduction takes place in the presence of palladium on charcoal and easily leads to a wide variety of α-amino vinylphosphonate compounds. The reduction of the same allenylphosphonates to allylphosphonates can be carried out by electrochemistry (Collaboration Pr. P. de Oliveira, Université Paris-Saclay). Therefore, from the same precursor, it is possible to chemoselectively obtain amino vinylphosphonates without traces of allylphosphonates and vice versa.
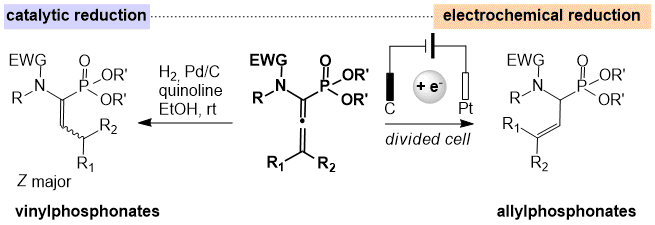
All these methodologies allow us to access the two regioisomers α- and β-amino vinylphosphonates from ynamides in order to be able to functionalize them later.
Subsequently, we wished to go further with these α-amino vinylphosphonates. We were interested in the synthesis of cyclic α-amino vinylphosphonates by using ring closing metathesis from acyclic α-amino vinylphosphonates. A mechanistic study was undertaken to prepare these highly functionalized and particularly congested compounds (Collaboration Dr. J. Prunet, University of Glasgow).
Our work on the synthesis of ynamides and the preparation of aminophosphonates allowed us to develop a simple and efficient synthesis of ynamido-phosphonates. These compounds, whose reactivity has not been explored yet, have proved to be very good partners for 1,3-dipolar cycloadditions in order to prepare isoxazoles.
Moreover, this study led us to form a large number of α-amino allenylphosphonates, new species whose reactivity and potential in synthesis remains underexplored. From para-methoxy α-amino allenylphosphonate derivatives we have carried out a 5-endo-dig type cyclizations in oxidizing conditions to form spirodienone lactams. These spiro motifs are present in many natural molecules of biological interest.
» Related papers
Highly Regio- and Stereoselective Nickel-Catalyzed Addition of Dialkyl Phosphites to Ynamides: an Efficient Synthesis of β-Aminovinylphosphonates. A. Fadel, F. Legrand, G. Evano, N. Rabasso, Ad. Synth. Catal., 2011, 353, 263-267
[2,3]-Sigmatropic Rearrangement of Ynamides: Preparation of α-Amino Allenephosphonates. F. Gomes, A. Fadel, N. Rabasso, J. Org. Chem., 2012, 77, 5439-5444
Selective Reduction of Amino Allenephosphonates: Preparation of α-Amino Vinylphosphonates. P. Adler, F. Gomes, A. Fadel, N. Rabasso, Eur. J. Org. Chem., 2013, 7546-7555
Strategies for the synthesis of α- and β-amino vinylphosphonate. P. Adler, A. Fadel, N. Rabasso, Tetrahedron, 2014, 70, 4437-4456
Cerium(iv) ammonium nitrate mediated 5-endo-dig cyclization of α-amino allenylphosphonates to spirodienones. P. Adler, A. Fadel, N. Rabasso, Chem. Commun., 2015, 51, 3612-3615
From acyclic to cyclic α-amino vinylphosphonates by using ring-closing metathesis. P. Adler, A. Fadel, J. Prunet, N. Rabasso, Org. Biomol. Chem., 2017, 15, 387-395
Synthesis of N-Sulfonyl Ynamido-Phosphonates: Valuable Partners for Cycloadditions. V. Perez, A. Fadel, N. Rabasso, Synthesis, 2017, 49, 4035-4044
Electrochemical Reduction of α-Amino Allenylphosphonates to α-Amino Allylphosphonates. P. Adler, P. De Oliveira, N. Rabasso, Eur. J. Org. Chem., 2020, 3918-3925
» Keywords associated with this part: Ynamides, Catalysis, Nickel, Sigmatropic Rearrangement, Reduction, Stereoselectivity, Cyclization, Metathesis, Electrosynthesis.
Preparation of Aminomethyl Vinylphosphonates: As part of our research on the reactivity of vinylphosphonates we have studied the synthesis of aminomethyl vinylphosphonates. These compounds are excellent partners for cycloaddition and 1,3-dipolar cycloaddition in particular.
» Related paper
Synthesis of new β- and γ-aminopyrrolidinephosphonates via 1,3-dipolar cycloaddition of substituted vinylphosphonates. N. Rabasso, A. Fadel, Tetrahedron Lett., 2010, 51, 60-63
» Keywords associated with this part: Cycloadditions.
Phosphonopeptide Synthesis: Since aminophosphonates are isosteres of aminoacids they represents the ideal condidats to modify and enhance the properties of peptides. Recently we have prepared an analogue of Met-enkephalin by replacement of methionine with our cyclic aminophosphonates.
» Related paper
Synthesis of an Enkephalin Analogue Containing an α,α-Disubstituted Heterocyclic Aminophosphonic Acid. N. Rabasso, A. Fadel, Phosphorus, Sulfur Silicon Relat. Elem., 2011, 186, 1811-1819
» Keywords associated with this part: Peptides, Enkephaniline, Kabachnik-Fields







