Chimie Inorganique
Fe complexes, bioinspired catalysts for oxidation
Axis 1 — ISOLATION AND STUDY OF REACTION INTERMEDIATES
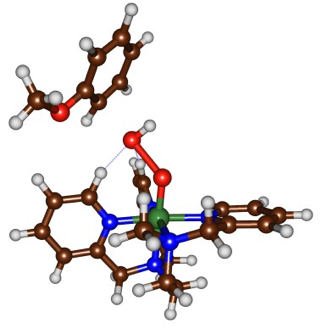
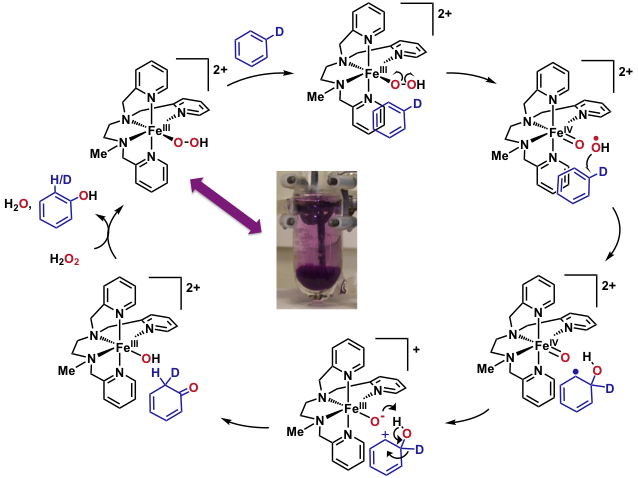
We have devised an experimental setup allowing us to generate and isolate reaction intermediates from FeII complexes and chemical oxidants at low temperature (T = -80°C). Subsequently, the intrinsic reactivity of these species (in the absence of the oxidant or its side products) can be studied. We have isolated for the first time a FeIII(OOH) intermediate in the solid state. The mechanistic study of its reaction with aromatic substrates under single turnover conditions revealed that the FeIII(OOH) undergoes an homolytic O-O clivage upon electrophilic attack on the substrate, which has been confirmed by DFT and Valence Bond methods (collaboration with Dr. S. P. de Visser, Univ. Manchester). The transition state for the reaction of FeIII(OOH) with anisole indeed shows an elongated O-O bond. Additionally, the spin density analysis on Fe and O atoms supports the nature of bond breaking. Calculations also confirm the experimentally observed inverse KIE and NIH shift.
Last update on 11.13.2019

