
|
Synthèse de Molécules et de Macromoléculespour le Vivant et l’Environnement |
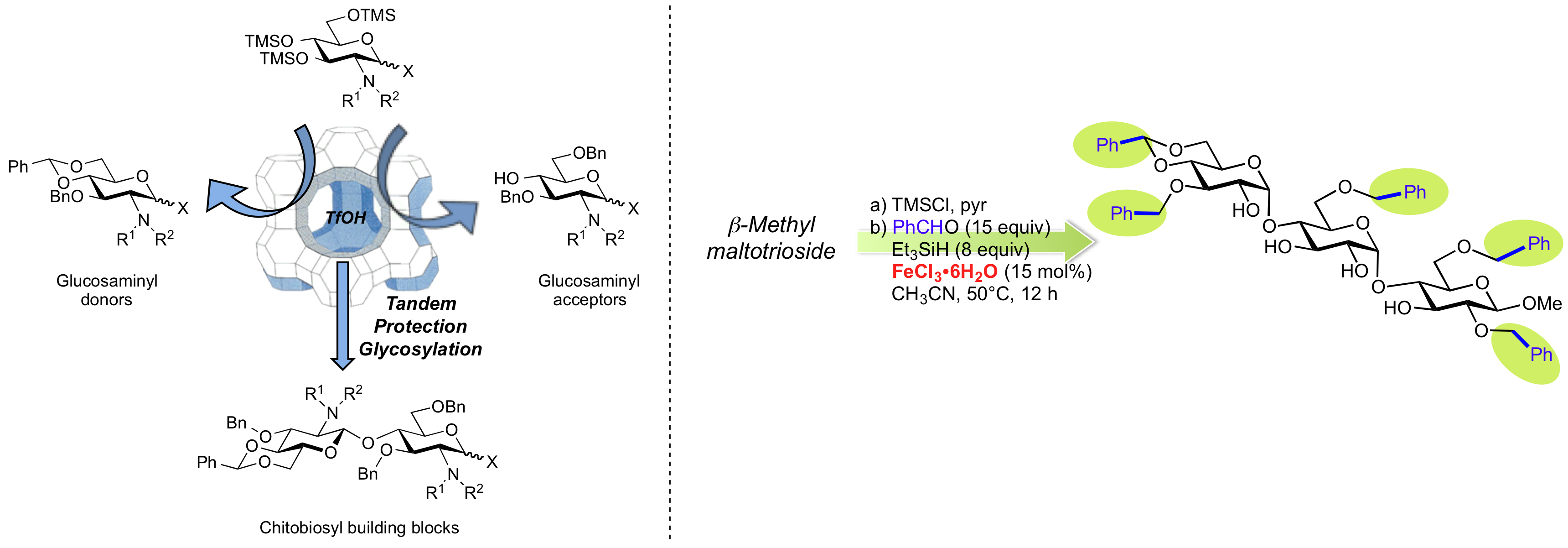
The discovery of atom-economical, catalytic one-pot multi-step processes for the chemo- and regioselective transformations of carbohydrates and polyols ("one catalyst in a single reaction vessel"). These transformations, avoiding time-consuming protection/deprotection steps and the isolation of intermediates, are catalyzed by copper(II) triflate or the environmentally friendly and inexpensive iron(III) chloride hexahydrate. This last catalyst was very efficient on disaccharides and one trisaccharide. Catalysis by triflic acid associated with powdered molecular sieves is a better option with the important the NHCO-containing functionalities on carbohydrates (e.g. derivatives of D-glucosamine). In addition, we demonstrated that chitin, source of the α-pentaacetate of N-acetyl glucosamine (GlcNAc) in one step, is the "bio-precursor" of simple GlcNAc α-glycosides by direct activation with catalytic amounts of copper(II) triflate in a heated sealed-vessel reactor. This methodological work also includes new developments in radical chemistry, such as the first evidence of a 1,7-hydride shift in a regioselective deprotection reaction on polyfunctional substrates.
Jean-Marie Beau, Yann Bourdreux, Guillaume Despras, Alexandra Gouasmat, Géraldine San Jose, Dominique Urban and Boris Vauzeilles, One-pot multi-step regioselective protection of carbohydrates catalyzed by acids, in Protecting Groups: Strategies and Applications in Carbohydrate Chemistry. Sébastien Vidal, Ed., Wiley, 2019, pp. 201-226. ISBN: 978-3-527-34010-1
From Chitin to Alpha-Glycosides of N-Acetylglucosamine Using Catalytic Copper Triflate in a Heated Sealed-Vessel Reactor Dany Frem, Dominique Urban, Stéphanie Norsikian, Jean-Marie Beau, Eur. J. Org. Chem. 2017, 34, 5094-5101.
Catalytic Iron(III) Chloride Mediates Site-Selective Protection of Mono- and Disaccharides and one Trisaccharide A. Gouasmat, A. Lemétais, J. Solles, Y. Bourdreux, J.-M. Beau, Eur. J. Org. Chem., 2017, 3355-3361
One-pot synthesis of D-glucosamine and chitobiosyl building blocks catalyzed by triflic acid on molecular sieves. G. Despras, D. Urban, B. Vauzeilles, J.-M. Beau, Chem. Commun. 2014, 50, 1067-1069
Jean-Marie Beau, Yann Bourdreux, François-Didier Boyer, Stéphanie Norsikian, Dominique Urban, Gilles Doisneau, Boris Vauzeilles, Alexandra Gouasmat, Aurélie Lemétais, Aurélie Mathieu, Jean-François Soulé, Arnaud Stevenin and Amandine Xolin, Chapter 7, “Recent results in synthetic glycochemistry with iron salts at Orsay-Gif” in Carbohydrate Chemistry: Volume 40, The Royal Society of Chemistry, 2014, 40, 118-139.
A Tin-Free Regioselective Radical De-O-benzylation by an Intramolecular Hydrogen Atom Transfer on Carbohydrate Templates. A. Attouche, D. Urban, J.-M. Beau, Angew. Chem. Int. Ed. 2013, 52, 9572-9575
Iron(III) chloride-tandem catalysis for a one-pot regioselective protection of glycopyranosides Bourdreux Yann, Lemétais Aurélie, Urban Dominique, Beau Jean-Marie Chem. Commun. 2011, 47, 2146-2148.
Tandem Catalysis for a One-Pot Regioselective Protection of Carbohydrates. The Example of Glucose Antoine Français, Urban Dominique, Beau Jean-Marie Angew. Chem. Int. Ed. 2007, 46, 8662-8665.
C-Glycosides
The development of a novel direct and chemoselective umpolung process on the carbohydrate glycals without relying on the preparation of intermediary pi-allyl transition metal complexes, with samarium diiodide as a simple reducing system. This proceeds with a high stereochemical outcome in the carbon-carbon bond forming step. It provided a new approach to the α-C-glycosides of the N-acetyl neuraminic acid. This single electron reductive metallation procedure was applied to the discovery of new selective hemagglutinin (HA) ligands and neuraminidase (NA) inhibitors targeting the binding or the catalytic site, respectively. Both glycoproteins are crucial for the viral infection by Influenza of type A virus (flu virus).

Direct Umpolung of Glycals Including 2,3-Unsaturated N-Acetylneuraminic Acid Derivatives Using Samarium Diiodide. X.-T. Le, C. Papin, G. Doisneau, J.-M. Beau, Angew. Chem. Int. Ed., 2014, 53, 6184-6187. VIP
Synthesis of spirolactonic C-sialosides induced by samarium diiodide. J. Pezzotta, D. Urban, R. Guillot, G. Doisneau, J.-M. Beau, Synlett, 2014, 25, 375-380
Fast Access to Robust C-Sialoside Multimers Papin Caroline, Doisneau Gilles, Beau Jean-Marie Chem. Eur. J. 2009, 15, 53-57.
Anomeric Samarium(III) Intermediates of N-Acetylneuraminic Acid from Anomeric 2-Pyridylsulfides Adeline Malapelle, Zouleika Abdallah, Doisneau Gilles, Beau Jean-Marie Heterocycles 2009, 77, 1417-1424.
Plant growth
The deconstruction of chitin, one of the most abundant renewable biopolymers, and reconstruction from fragments to highly bioactive glycoconjugates and glycoprobes. It has been used in the total synthesis of the Nodulation and Mycorrhizal Factors and some mimetic structures for open-field tests (in collaboration with Bayer CropScience). The elaboration of photoactivatable glycolipid probes has also been very useful for the first identification of the long-elusive nodulation factor receptor.

From Chitin to Bioactive Chitooligosaccharides and Conjugates: Access to Lipochitooligosaccharides and the TMG-chitotriomycin Guillaume Despras, Aurélien Alix, Dominique Urban, Boris Vauzeilles, Jean-Marie Beau, Angew. Chem. Int. Ed. 2014, 53, 11912-11916.
Efficient chemoenzymatic synthesis of lipo-chitin oligosaccharides as plant growth promotors Rémi Chambon, Guillaume Despras, Antoine Brossay, Boris Vauzeilles, Dominique Urban, Jean-Marie Beau, Sylvie Armand, Sylvain Cottaz, Sébastien Fort, Green Chem. 2015, 17, 3923-3930.
Lipo-Chitooligosaccharidic Nodulation Factors: Synthesis and Agricultural Perspectives J.-M. Beau, Chimia, 2011, 65, 45-48.
Synthesis of complex glycolipids
The catalytic one-pot multi-step processes using the iron catalyst was extended to the trehalose disaccharide for the synthesis of novel highly antigenic sulfoglycolipids. Increasing the length of the chiral 1,3-methyl branch fatty acid of mycobacterial diacylated sulfoglycolipid analogues induces a dramatic improvement in their antigenic properties, giving products more potent than the natural antigens. These constructs should make excellent candidates as components of sub-unit vaccines. This project also involves the preparation of imaging probes based on trehalose and mycolic acids for the study of the mycomembrane biogenesis in mycobacterium and related organisms.
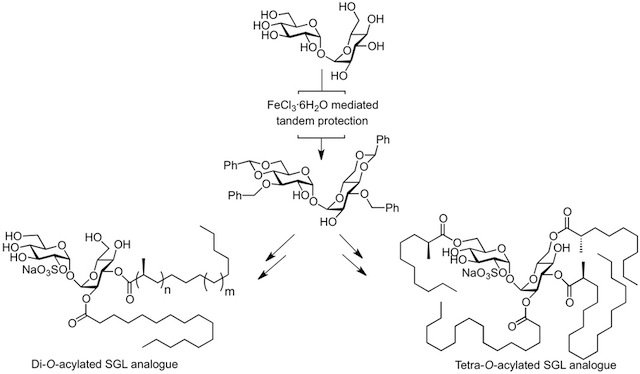
"Synthesis of a Mycobacterium tuberculosis Tetra-acylated Sulfolipid Analogue and Characterization of the Chiral Acyl Chains Using Anisotropic NAD 2D-NMR Spectroscopy" A. Lemétais, Y. Bourdreux, P. Lesot, J. Farjon, J.-M. Beau, J. Org. Chem. 2013, 78, 7648–7657.
"Simplified Deoxypropionate Acyl Chains for Mycobacterium tuberculosis Sulfoglycolipid Analogues: Chain Length is Essential for High Antigenicity" B. Gau, A. Lemétais, M. Lepore, L. F. Garcia-Alles, Y. Bourdreux, L. Mori, M. Gilleron, G. De Libero, G. Puzo, J.-M. Beau, J. Prandi, ChemBioChem 2013, 14, 2413–2417.
"Recent results in synthetic glycochemistry with iron salts at Orsay-Gif" J.-M. Beau, Y. Bourdreux, F.-D. Boyer, S. Norsikian, D. Urban, G. Doisneau, B. Vauzeilles, A. Gouasmat, A. Lemétais, A. Mathieu, J.-F. Soul., A. Stévenin, A. Xolin, in Carbohydrate Chemistry, vol. 40 (Eds.: A. P. Rauter, T. K. Lindhorst, Y. Queneau), The Royal Society of Chemistry, Cambridge, UK, 2014, pp. 118–139.
For the synthesis of native trehalose monomycolate and trehalose-based probes for the study of the mycomembrane, see:
Synthesis of chemical tools to label the mycomembrane of corynebacteria using a modified Iron (III) chloride-mediated protection of trehalose. M. Carlier, E. Lesur, A. Baron, A. Lemétais, K. Guitot, L. Roupnel, C. Dietrich, G. Doisneau, D. Urban, N. Bayan, J.-M. Beau, D. Guianvarc’H, B. Vauzeilles, Y. Bourdreux, Org. Biomol. Chem., 2022, 20, 1974-1981
Etudier la membrane des mycobactéries via une approche chémobiologique. E. Lesur, Y. Bourdreux, L'Actualité Chimique, 2021, 465, 63-64
"First access to a mycolic acid-based bioorthogonal reporter for the study of the mycomembrane and mycoloyltransferases in Corynebacteria". E. Lesur, A. Baron, C. Dietrich, M. Buchotte, G. Doisneau, D. Urban, J.-M. Beau, N. Bayan, B. Vauzeilles, D. Guianvarc’h, Y. Bourdreux, Chem. Commun., 2019, 55, 13074-13077
"Study of the conformational behaviour of trehalose mycolates by FT-IR spectroscopy". F. Migliardo, Y. Bourdreux, M. Buchotte, G. Doisneau, J.-M. Beau, N. Bayan, Chem. Phys. Lipids, 2019, 223, 104789

