Méthodologie, Synthèse et Molécules Thérapeutiques
Amino Acids and Peptides
This project is divided in 2 topics: development of original asymmetric synthesis of quaternary α-amino acids by memory of chirality (MOC); synthesis of β,γ-diamino acids and their use to build γ-peptides.
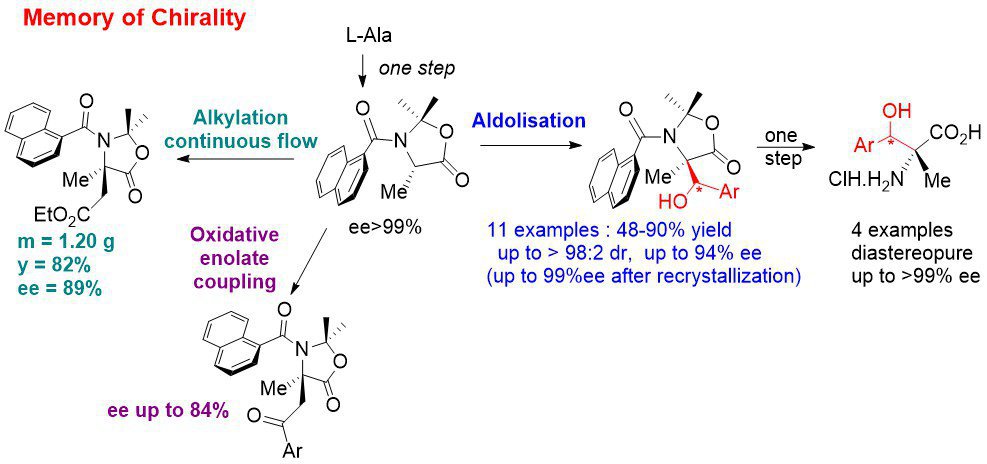
Memory of chirality: In continuation of our previous work, we have developed an asymmetric synthesis of β-hydroxy quaternary α-amino acids by MOC aldolisation. This means that this synthesis uses only the chirality of the starting α-amino acids to access to highly enantioenriched quaternary α-amino acids even though the initial stereogenic center is temporarily destroyed. The chirality is memorized by a chiral conformation of tertiary aromatic amide. Recently, we have also adapted our MOC alkylation reaction to continuous flow in collaboration with Pr. J.-i. Yoshida (Kyoto University, Japan). We are currently working on other types of reactions by MOC: oxidative coupling of enolates (radical reactions) and 1,3-dipolar cycloadditions.
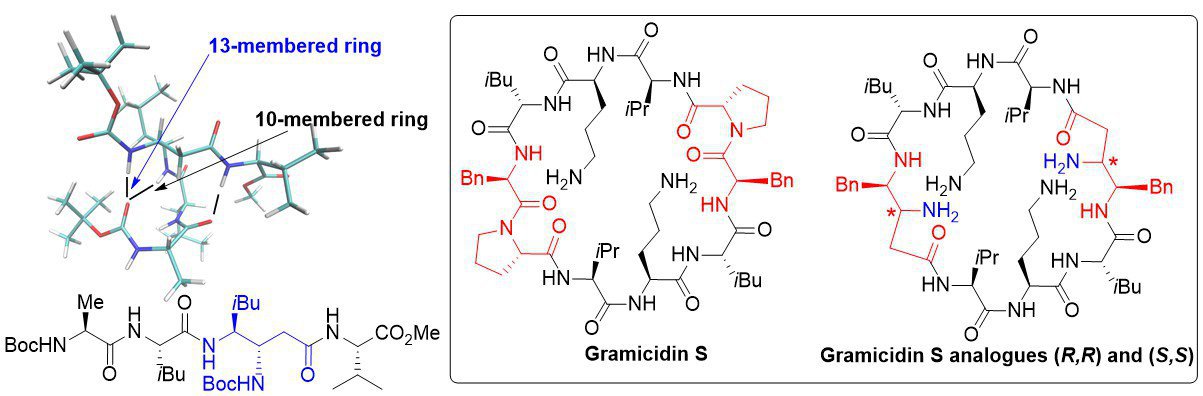
β,γ-diamino acids and γ-peptides: We have used β,γ-diamino acids to build original peptidic structures. We have thus shown that a tetrapeptide adopted a folded structure stabilized by several hydrogen bonds, involving the β-nitrogen of the β,γ-diamino acid. We have also synthesized and tested (collaboration with S. Zirah) the antimicrobial activities of analogues of Gramicidin S. Our analogues are less active than the parent peptide but present almost no haemolytic activity.