Inorganic Chemistry
Magnetic nanosystems
Axis 5. Redox-active magnetic molecules
Talal Mallah, Nathalie Bridonneau, François Lambert
PhD students: Dr. Yiting Wang, Aristide Colin, Solène Delaporte
Collaborations:
- Nicolas Suaud, Nathalie Guihéry, Hélène Bolvin (études théoriques, Laboratoire de Chimie et Physique Quantiques, Toulouse)
- Zakaria Halime (spectroéléctrochimie, ECI ICMMO)
- Jérôme Cornil, Imane Arbouch (études théoriques, Université de Mons, Belgique)
- ShinIchi Ohkoshi (Ohkoshi Laboratory, Université de Tokyo, Japon)
Related publications:
- Y. Wang, F. Lambert, E. Rivière, R. Guillot, C. Herrero, A. Tissot, Z. Halime, T. Mallah, Chemical Communication, 55, 12336-12339, 2019
- N. Suaud,
A. Colin, M. Bouammali, T. Mallah, N. Guihéry, Chem. Eur. J. 2023, e202302256. https://doi.org/10.1002/chem.202302256
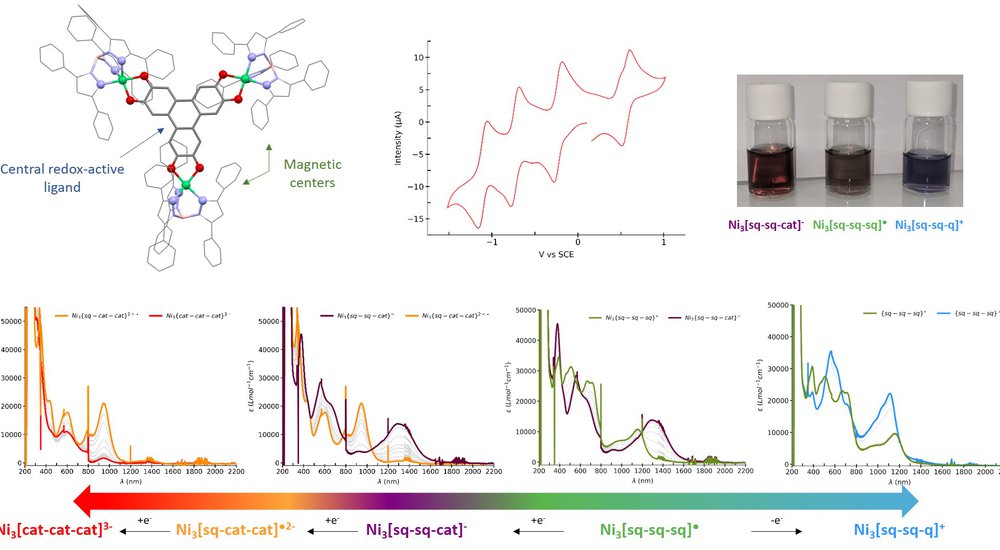
This work focuses on the rational design of magnetic systems (qubits, molecule-magnets) based on transition metals with the aim of controlling interactions between metal ions using a central redox active ligand. We use a central ligand that has three positions available for complexation, each of which can switch between three redox states: quinone/semiquinone/cathecol. We have isolated a trinuclear [Ni3] complex in which all Ni(II) ions are pentacoordinated and the central ligand is in the {sq-sq-sq} state. Cyclic voltammetry shows four reversible monoelectron waves (three in reduction, one in oxidation), which defines five potentially accessible redox states for this complex. Spectroelectrochemical measurements allowed the characterization of these states, and we were able to isolate and characterize the first oxidized {sq-sq-q} and reduced {sq-sq-cat} states in the solid state (powders or crystals).
Electron paramagnetic resonance (EPR) and SQUID magnetometry measurements show that the magnetic interaction between spin carriers is influenced by the electronic state of the central ligand. Obtaining crystal structures allows us to precisely define the presence and location of radicals which is not the same in the different redox states, and thus to rationalize the observed magnetic behavior. Additionally, ab initio calculations provide information on the electronic structure of the studied species. Specifically, the properties of the central redox active ligand are examined and help explain the experimentally observed properties of the complexes. We are currently trying to understand and rationalize the reciprocal interaction between the redox-active ligand, the nature of the metallic center (Ni, Co), and the influence of the external ligand. Overall, this redox-active magnetic system is rich and opens the way to a possible switching of different magnetic systems (qbit, valence tautomerism, molecule-magnet).