 Chimie Peptidomimétique Photochimie et Procédés Alternatifs
Chimie Peptidomimétique Photochimie et Procédés Alternatifs
Responsable : Marie-Christine Scherrmann
Mail : marie-christine.scherrmann@universite-paris-saclay.fr
Tél. : +33 1 69 15 72 56
Heterogeneous asymmetric organocatalysis
We have developed a novel heterogeneous chiral catalyst by grafting cupreine onto amorphous silica. Cupreine (CPN) was readily prepared in one step from commercially available quinine then grafted to the silica support through a thioether bond involving the 3-mercaptopropyl triethoxysilane (MPTES) as a linker between the catalyst and silica.
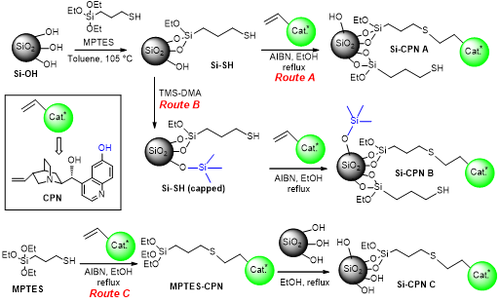
Our results attested for the first time that silica is a suitable support to immobilise CPN while retaining the asymmetric catalytic performance of this bifuctional catalyst almost unchanged for the asymmetric Michael addition of malonate to trans-beta-nitrostyrene in some biomass-derived solvents.
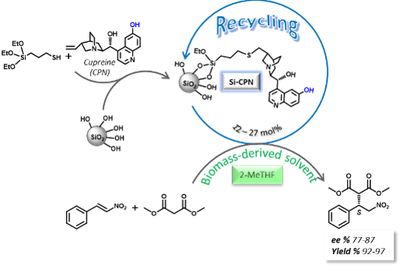
reference: Cupreine grafted onto silica as an enantioselective and recyclable catalyst for the 1,4-addition of malonate to trans-beta-nitrostyrene, I. Billault, R. Launez and M.-C. Scherrmann, RSC Adv., 2015, 5, 29386–29390. DOI: 10.1039/c5ra02292d
We are now investigating the use of Si-CPN for various applications under continuous flow conditions.

