 Chimie Peptidomimétique Photochimie et Procédés Alternatifs
Chimie Peptidomimétique Photochimie et Procédés Alternatifs
Responsable : Marie-Christine Scherrmann
Mail : marie-christine.scherrmann@universite-paris-saclay.fr
Tél. : +33 1 69 15 72 56
Organic synthesis using photochemistry
In the early 2000s, we began working on four-membered rings and in particular we were interested in the ability of small-ring β-amino acids to induce particular conformational preferences in peptides (see this page). Six- and five-membered carbocyclic β-amino acids were already attracting considerable interest, yet the four-membered ring equivalents – derivatives of 2-aminocyclobutane-1-carboxylic acid (ACBC) – had not received the same attention, mainly due to the lack of convenient synthetic methods for the preparation of these molecular building blocks. Photochemical [2+2] cycloadditions involving nucleic bases were well known to biochemists, notably due to the deleterious effects that such reactions can have on DNA. However, organic chemists had not up to that point considered pyrimidinediones as photochemically-active enones having significant applications in preparative synthetic procedures. We therefore embarked on the objective of elaborating a photochemical strategy for the synthesis of the ACBC core structure, based on the [2+2] cycloaddition reaction of heterocyclic enones and simple alkenes.
This project has evolved progressively to consider other heterocyclic enone partners and to allow access to heterocyclic β-amino acids and cyclobutane γ-amino acids too, and has also led us to examine other interesting examples of photochemical transformations of organic molecular architectures.
All of the reactions described in these pages are conducted on preparative scale, confirming that photochemistry is indeed a valuable addition to the synthetic chemist’s toolbox.
-
PHOTOCHEMICAL SYNTHESIS OF CYCLOBUTANE β-AMINOACIDS
The core reaction in our early work was the facile [2+2] photocycloaddition reaction between uracil and ethylene. We used this reaction to provide a simple access to ACBC, the parent cyclobutane β-aminoacid. Over the last decade various means of controlling the stereochemistry have been examined and today this synthetic strategy represents the most convenient construction of any stereoisomer of this building block. Amino Acids 2011, 41, 587; J. Org. Chem. 2009, 74, 3217; Synthesis 2007, 2222; Tetrahedron Lett., 2004, 45, 7095; Tetrahedron Lett. 2002, 43, 6177.
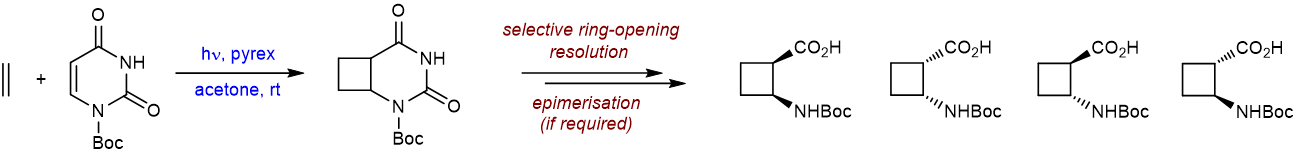
The reaction can be applied using a selection of 5- or 6-substituted uracils, allowing the preparation of C1 or C2-substituted ACBC derivatives. In a recent example, a cis-ACBC derivative with a fluorine on the C1 position was required; this material was prepared easily in enantiomerically pure form.. New J. Chem., 2015, 39, 3270; Synlett 2006, 1394.

In order to prepare C3 or C4-substituted ACBCs, a different enone was used: maleic anhydride is a convenient partner for [2+2] photochemical cycloaddition reactions with non-symmetrical unsaturated hydrocarbons. In this strategy, the downstream selective transformations of the carboxylate motifs is the key for the controlled construction of the ACBC feature.. Org. Biomol. Chem. 2014, 12, 8212.

-
PHOTOCHEMICAL SYNTHESIS OF HETEROCYCLIC 4-MEMBERED RING β-AMINOACIDS
The [2+2] photocycloaddition reaction also proceeds with 6-azauracil, providing an entry to the previously unknown aza-analogue of ACBC. Again, both enantiomers can be obtained through chiral resolution. J. Org. Chem. 2011, 76, 708.

The cis-oxetane β-aminoacid is a natural product known as oxetin. We revisited a Paternò−Büchi [2+2] photocycloaddition approach, first considered by T. Bach, to prepared all four stereoisomers of this unusual molecule. J. Org. Chem. 2016, 81, 9983.

-
PHOTOCHEMICAL SYNTHESIS OF CYCLOBUTANE γ-AMINOACIDS
Conformationally-restricted analogues of the neurotransmitter γ-aminobutyric acid (GABA) are of considerable interest. In order to prepare a GABA analogue with a cyclobutane ring backbone restriction at Cα–Cβ, an unsaturated γ-lactam bearing a chiral appendage was engaged in a [2+2] photocycloaddition reaction with ethylene. All four stereoisomers of 2-(aminomethyl)cyclobutane-1-carboxylic acid were accessed via this route. Tetrahedron, 2013, 69, 3571; Tetrahedron Lett., 2011, 52, 1253.

To prepare a GABA analogue with a cyclobutane ring backbone restriction at Cβ–Cγ, a different photochemical approach was used: an azepin-2-one underwent an electrocyclization reaction to close the four-membered ring. After reduction, hydrolysis and resolution both enantiomers of cis-(2-aminocyclobutyl)acetic acid were obtained. We have also performed an enantioselective version of this synthesis using a chiral host–guest approach in the solid state. J. Org. Chem. 2017, 82, accepted article (DOI 10.1021/acs.joc.7b01300); Eur. J. Org. Chem. 2014, 7148.

-
PUBLICATIONS
β-Cyclodextrin-mediated enantioselective photochemical electrocyclization of 1,3-dihydro-2H-azepin-2-one. A. T. MANSOUR, J. BUENDIA, J. XIE, F. BRISSET, S. ROBIN, D. NAOUFAL, O. YAZBECK, D. J. AITKEN, J. Org. Chem., 2017, 82, 9832-9836
Synthetic access to all four stereoisomers of oxetin. A. F. KASSIR, S. S. RAGAB, T. A. M. NGUYEN, F. CHARNAY-POUGET, R. GUILLOT, M.-C. SCHERRMANN, T. BODDAERT, D. J. AITKEN, J. Org. Chem., 2016, 81, 9983-9991.
Conformational preferences in the b-peptide oligomers of cis-2-amino-1-fluorocyclobutane-1-carboxylic acid. A. HASSOUN, C. M. GRISON, R. GUILLOT, T. BODDAERT, D. J. AITKEN, New J. Chem., 2015, 39, 3270-3279.
Practical syntheses of both enantiomers of the conformationally restricted GABA analogue cis-(2-aminocyclobutyl)acetic acid. H. AWADA, S. ROBIN, R. GUILLOT, O. YAZBECK, D. NAOUFAL, N. JABER, A. HACHEM, D. J. AITKEN, Eur. J. Org. Chem., 2014, 7148-7155.
Stereoselective intermolecular [2 + 2]-photocycloaddition reactions of maleic anhydride: stereocontrolled and regiocontrolled access to 1,2,3-trifunctionalized cyclobutanes. F. HERNVANN, G. RASORE, V. DECLERCK, D. J. AITKEN, Org. Biomol. Chem., 2014, 12, 8212-8222.
Photochemical transformation of a 1,2-dihydropyridin-3-one: an original tandem retro-[4+ 2] / [2+2] cycloaddition process. D. J. AITKEN, A FRONGIA, X. GAUCHER, J. OLLIVER, H. RAFIQUE, C. SAMBIAGIO, F. SECCI, Tetrahedron Lett., 2013, 54, 2825-2827.
Molecular structures of the photodimers of 5-phenyluracil and 6-phenyluracil. A VIDAL, R. PAUGAM, S. FAURE, E. PEREIRA, D. J. AITKEN, Tetrahedron Lett., 2013, 54, 2536-2537.
A unified synthesis of all stereoisomers of 2-(aminomethyl)cyclobutane-1-carboxylic acid. V. ANDRE, M. GRAS, H. AWADA, R. GUILLOT, S. ROBIN, D. J. AITKEN, Tetrahedron, 2013, 69, 3571-3576.
Photochemical synthesis of four-membered ring amino acids. D. J. AITKEN, European Photochemistry Association Newsletter, December 2012, 34-37.
Rapid access to cis-cyclobutane g-amino acids in enantiomerically pure form. V. ANDRE, A. VIDAL, J. OLLIVIER, S. ROBIN, D. J. AITKEN, Tetrahedron Lett., 2011, 52, 1253-1255.
A refined synthesis of enantiomerically pure 2-aminocyclobutane carboxylic acids. V. DECLERCK, D. J. AITKEN, Amino Acids, 2011, 41, 587-595.
endo-6-(Hydroxymethyl)bicyclo[3.2.0]hept-3-en-2-one esters and the photochemical challenge: [2+2] cycloaddition versus skeletal rearrangement. M. LE LIEPVRE, J. OLLIVIER, D. J. AITKEN, Tetrahedron: Asymmetry, 2010, 21, 1480-1485.
Expedient preparation of all isomers of 2‑aminocyclobutane-1-carboxylic acid in enantiomerically pure form. C. FERNANDES, E. PEREIRA, S. FAURE, D. J. AITKEN, J. Org. Chem., 2009, 74, 3217-3220.
Synthesis of functional bicyclo[3.2.0]heptanes: a study of the [2+2] photocycloaddition reactions of 4‑hydroxycyclopent-2-enone derivatives M. LE LIEPVRE, J. OLLIVIER, D. J. AITKEN, Eur. J. Org. Chem., 2009, 5953-5962.
Efficient synthesis of 3-hydroxymethylated cis- and trans-cyclobutane b-amino acids using an intramolecular photocycloaddition strategy. A. MONDIÈRE, R. PENG, R. REMUSON, D. J. AITKEN, Tetrahedron, 2008, 64, 1088-1093.
Photochemical behaviour of 5-formyl and 5-acetyl uracils in the presence of ethene. E. PEREIRA, S. FAURE, D. J. AITKEN, Tetrahedron Lett., 2008, 49, 1968-1970.
Synthesis of (+)-coniceine via reductive photocyclization of dienamides: an entry to indolizidines. T. HJELMGAARD, D. GARDETTE, D. TANNER, D. J. AITKEN, Tetrahedron: Asymmetry, 2007, 18, 671-678.
[2+2] Photocycloadditions with chiral uracil derivatives: access to all four stereoisomers of 2‑aminocyclobutanecarboxylic acid. C. FERNANDES, C. GAUZY, Y. YANG, O. ROY, E. PEREIRA, S. FAURE, D. J. AITKEN, Synthesis, 2007, 2222-2232.
A solution to the component instability problem in the preparation of peptides containing C2-substituted cis-cyclobutane b-aminoacids: synthesis of a stable Rhodopeptin analogue. O. ROY, S. FAURE, D. J. AITKEN, Tetrahedron Lett., 2006, 47, 5981-5984.
Synthesis of the constrained glutamate analogues (2S,1′R,2′R)- and (2S,1′S,2′S)-2-(2′-carboxy-cyclobutyl)glycines L-CBG-II and L-CBG-I by enzymatic transamination. X. GU, M. XIAN, S. ROY-FAURE, J. BOLTE, D. J. AITKEN, T. GEFFLAUT, Tetrahedron Lett., 2006, 47, 193-196.
The [2+2] photocycloaddition of uracil derivatives with ethylene as a general route to cis-cyclobutane b-aminoacids. C. GAUZY, B. SABY, E. PEREIRA, S. FAURE, D. J. AITKEN, Synlett, 2006, 1394-1398.
Synthesis of (+)-(1S,2R) and (–)-(1R,2S)-2-aminocyclobutane-1-carboxylic acids. C. GAUZY, E. PEREIRA, S. FAURE, D. J. AITKEN, Tetrahedron Lett., 2004, 45, 7095-7097.
A short synthesis of the cis-cyclobutane b-aminoacid skeleton using a 2+2 cycloaddition strategy. D. J. AITKEN, C. GAUZY, E. PEREIRA, Tetrahedron Lett., 2002, 43, 6177-6179.

