 CP3A ORGANIC SYNTHESIS GROUP
CP3A ORGANIC SYNTHESIS GROUP
Peptidomimetic Chemistry, Photochemistry, Alternative Processes
Chemistry in alternative solvents

Total synthesis of triazole-linked C-glycosyl flavonoids in alternative solvents and environmental assessment in terms of reaction, workup and purification
An efficient total synthesis of triazole-linked C-glycosylflavonoids was developed without the use of protective groups in order to promote atom economy, employing alternative solvents, and choosing the least toxic reagents. Aiming to measure the impact of the operation that affects the mass intensity to a greater extent, we envisaged first determining this green metric for the reaction (MIR), the workup (MIW), and the purification (MIP) for each step and then taking advantage of these values to calculate the contributions to the total synthesis (see "Process evaluation tools" section).
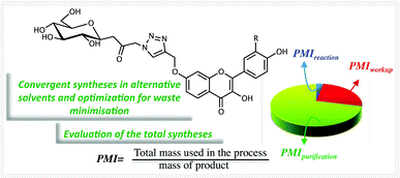
reference: Total synthesis of triazole-linked C-glycosyl flavonoids in alternative solvents and environmental assessment in terms of reaction, workup and purification. F. Pessel, I. Billault and M.-C. Scherrmann, Green Chem., 2016, 5558-5568. DOI: 10.1039/c6gc01647b
Total synthesis of high loading capacity PEG-based supports
The aim of this work was the synthesis of high loading capacity polymers by implementing methods of green chemistry. Replacement of solvents, traditionally used in organic synthesis, by recyclable alternative media such as PEG400 together with the use of purification methods such as ultrafiltration of aqueous media, where possible, also contributed to the eco-compatible synthesis.
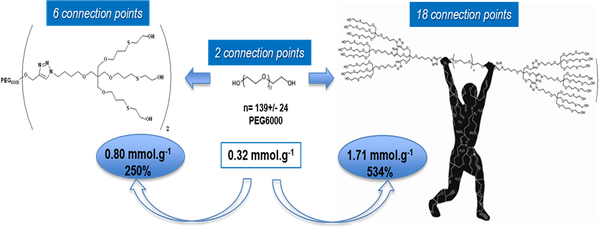
The new polymer featuring 6 connection points has a loading capacity of 0.80 mmol/g whereas that with 18 connection points has a loading capacity of 1.71 mmol/g, which correspond to an increase of 250% and 534%, respectively, with respect to the starting PEG6000. See also “Evaluation of processes by green metrics”
reference: Total synthesis of high loading capacity PEG-based supports: evaluation and improvement of the process by use of ultrafiltration and PEG as a solvent. R. Turgis, I. Billault, S. Acherar, J. Augé, M-C. Scherrmann, Green Chem., 2013, 1016-1029. DOI:10.1039/c3gc37097f
PEG as solvent
The copper-catalysed azide–alkyne cycloaddition was investigated in molten PEG2000 and PEG400 using various sources of Cu(I). The reaction proceeded smoothly and afforded the corresponding triazole in good yields. In PEG the reaction could be carried out under an uncontrolled atmosphere. Depending on the solubility of the product, different types of treatments were investigated:
- Ultrafiltration in water (PEG2000)
- Precipitation of the polymer-solvent (PEG2000)
- Liquid-liquid extraction (PEG400)
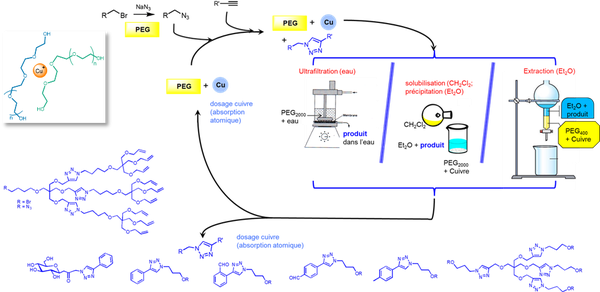
reference: Investigation of the copper(I) catalyzed azide–alkyne cycloaddition reactions (CuAAC) in molten PEG2000, Isabelle Billault, Freddy Pessel, Alain Petit, Raphaël Turgis and Marie-Christine Scherrmann, New J. Chem., 2015, 39, 1986-1995. DOI: 10.1039/c4nj01784f.
PEG as water soluble support
PEG-supported aqueous flow synthesis coupled with ultrafiltration as the separation technique has been investigated for the first time. This strategy was applied to the preparation of new 3,4-dihydropyrimidin-2(1H)-ones, tetrazoles and tetrahydro-1,3-oxazines from the same PEG-linked aldehyde as case studies. In order to improve the NMR characterization of PEG-branched products, we developed new NMR experiments named SENSitivity increAsed and resolution enhanced by Signal Suppression or SENSASS NMR.
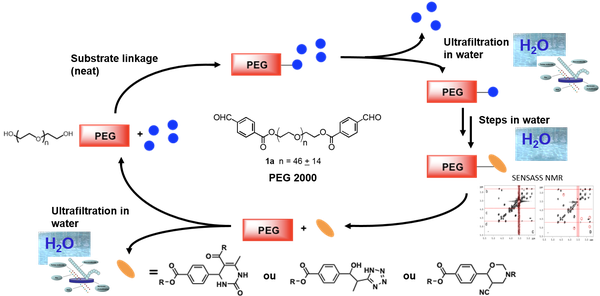
See also " flow chemistry"
references:
Soluble polymer-supported flow synthesis: A green process for the preparation of heterocycles, Nicolò Prosa, Raphaël Turgis, Riccardo Piccardi, and Marie-Christine Scherrmann, Eur. J. Org. Chem. 2012, 2188–2200. DOI: 10.1002/ejoc.201101726.
SENSASS NMR: New NMR techniques for enhancing the sensitivity and the spectral resolution of polymer supported chemicals, Nicolo Prosa, Marie-Christine Scherrmann, Denis Merlet, and Jonathan Farjon, J. Magn. Reson., 2013, 237, 63–72. DOI: 10.1016/j.jmr.2013.09.011.

